
Can the natural process of aging wine in corked bottles be accelerated?
I recently found an interesting article on how an electric field can be used for maturation of wine (New Scientist news coverage of the article). Applying a AC field of 600 V/cm for 3 minutes resulted in an accelerated aging of wine and according to the authors of the paper, it made “harsh and pungent raw wine become harmonious and dainty”. They observed changes in concentrations of higher alcohols, aldehydes, esters and free amino acids. But I was quite surprised that they don’t say anthing about astringency and polyphenols (tannins). I’d expect some changes there as well, but alas it’s so much more difficult to measure the polyphenols than the low molecular compounds. A sensory panel identified both positive and negative effects of the electric treatment which helped identify an optimum treatment. Apparently several Chinese wine manufacturers are testing the technology on a pilot scale now. Many people have a romantic impression of how wine is made, but the extensive catalogues of “corrective chemicals” available to the modern wine maker should perhaps make you reconsider the romatic idea of wine making. Even professor Hervé Alexandre at the University of Burgundy has given the technology a thumbs up: “Using an electric field to accelerate ageing is a feasible way to shorten maturation times and improve the quality of young wine”. Who knows – maybe you’ll soon be drinking a wine that has been zapped?
 Moving from industrial scale wine upgrading to kitchen scale gadgets: In his latest “curious cook” column Harold McGee writes about different gadgets that supposedly can change the flavor of wines. To the better of course. He mentions the Wine wand which is supposed to speed up aeration of wines. The promotional explanation on the web page sounds quite dubious, take for instance the claim that the wine wand can “accelerate the aerating process of wine by replicating the natural frequencies of air and oxygen, and infusing them into the wine”. Complete nonsense! Harold McGee however mentions that he did several blind tests and found that there were differences. I guess we can’t exclude the possiblity that there could be some kind of reactive surface on these wands. From the pictures there seem to be some small (glass?) beads in a hollow cylinder. I can’t find any information about the surface. Perhaps it’s been activated or coated with a metal? In that case we could have plenty of surface chemistry going on. If it’s only glass however – well – then I’d just leave the wine to mature in it’s glass bottle.
Moving from industrial scale wine upgrading to kitchen scale gadgets: In his latest “curious cook” column Harold McGee writes about different gadgets that supposedly can change the flavor of wines. To the better of course. He mentions the Wine wand which is supposed to speed up aeration of wines. The promotional explanation on the web page sounds quite dubious, take for instance the claim that the wine wand can “accelerate the aerating process of wine by replicating the natural frequencies of air and oxygen, and infusing them into the wine”. Complete nonsense! Harold McGee however mentions that he did several blind tests and found that there were differences. I guess we can’t exclude the possiblity that there could be some kind of reactive surface on these wands. From the pictures there seem to be some small (glass?) beads in a hollow cylinder. I can’t find any information about the surface. Perhaps it’s been activated or coated with a metal? In that case we could have plenty of surface chemistry going on. If it’s only glass however – well – then I’d just leave the wine to mature in it’s glass bottle.
 The other object he mentions is the Clef du Vin or wine key which is more interesting from a chemical perspective. The active part consists of a metal disc which (in a preferred embodiment to quote the patent jargon) consists of 95% copper, 3% gold and 2% silver. According to the description in the patent application, the device is capable of an “accelerated and gauged oxidation-reduction of the wine”. Dipping the disc into a glas of wine for one second is supposed to equal one year of cellar aging. Metals can catalyze many reactions, and there are many reactive compounds in wine so I wouldn’t be surprised if something happens. Considering the fact that sulfurous compounds (such as hydrogensulfide for instance) are very potent, and that sulfur has an affinity to several metals such as gold, copper and silver it seems plausible that the metal disc may actually remove some sulfides from the wine by adsorption and in turn influence the flavor. However, in the course of one second only a small fraction of the wine has been in contact with the metal disc, so I can’t really see how this should be sufficient. It would in a way be strange if only desirable reactions are catalyzed (i.e. only undesirable compounds are degraded/removed). Anyhow – I’d really like to see a peer reviewed paper on this. For someone with spare time and access to a GC-MS this should be a nice project 🙂
The other object he mentions is the Clef du Vin or wine key which is more interesting from a chemical perspective. The active part consists of a metal disc which (in a preferred embodiment to quote the patent jargon) consists of 95% copper, 3% gold and 2% silver. According to the description in the patent application, the device is capable of an “accelerated and gauged oxidation-reduction of the wine”. Dipping the disc into a glas of wine for one second is supposed to equal one year of cellar aging. Metals can catalyze many reactions, and there are many reactive compounds in wine so I wouldn’t be surprised if something happens. Considering the fact that sulfurous compounds (such as hydrogensulfide for instance) are very potent, and that sulfur has an affinity to several metals such as gold, copper and silver it seems plausible that the metal disc may actually remove some sulfides from the wine by adsorption and in turn influence the flavor. However, in the course of one second only a small fraction of the wine has been in contact with the metal disc, so I can’t really see how this should be sufficient. It would in a way be strange if only desirable reactions are catalyzed (i.e. only undesirable compounds are degraded/removed). Anyhow – I’d really like to see a peer reviewed paper on this. For someone with spare time and access to a GC-MS this should be a nice project 🙂

A stainless steel “soap” is believed to remove garlic stains from your fingers
Interestingly there is a totally different product that relies on the same chemistry: the steel soap. It is typically shaped like a standard soap bar and consists of plain normal stainless steel. It’s supposed to remove garlic, onion and fish smell from your fingers. It works by rubbing your hands against it under running water. I have one, but to be honest it’s hard to really say if it works or not – perhaps some have more experience with it? I had a friend of mine analyze my stainless steel soap by XPS and he gave me the following elemental composition for the six most abundant elements: 70.6% iron, 18.5% chromium, 8.2% nickel, 1.4% manganese, 0.7% molybdenum and 0.3% copper. This is more commonly known as 18/8 steel where 18 denotes 18% chromium and 8 denotes 8% nickel and it’s what all your forks and knives and other stainless steel tools are made of (which of course means that just about any stainless steel object you have in the kitchen should serve the purpose to remove odor from your fingers). Of the metals present here molybdenum in particular is used industrially for desulfurization of oil. Based on a paper on hydrodesulfurization I speculate whether the mechanism could be something like this:
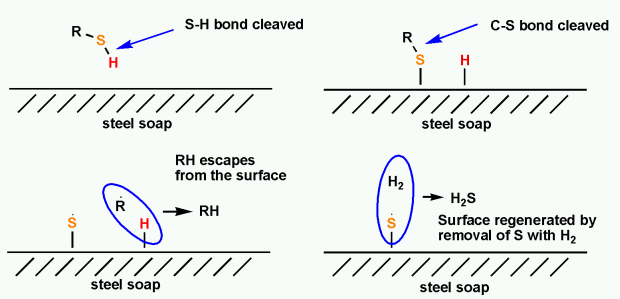
A proposed mechanism for desulfurization on the surface of a “steel soap”
A sulfur compound exemplified here with a thiol (R-SH) reacts with the steel soap surface and the S-H bond is cleaved. Then the S-C bond is cleaved homolytically to yield radical species. The alkyl radical abstracts hydrogen from the surface and escapes whereas sulfur remains bound to the surface. The surface could be regenerated by removal of sulfur with hydrogen. All in all the chemistry of a steel soap seems plausible to me, but I’m not sure whether the effect is significant effect when it comes to removing that garlic smell from my fingers.

I’ve used stainless steel for years to remove garlic odors from my hands, and to my nose it definitely works. I never bought one of those “soap” gizmos – I just use a spoon or such.
I’ve read that plastic wrap can be used to remove the taint from corked wine. I’ve been wanting to try this, but I fortunately haven’t had a corked bottle for some time! Has anyone tried this trick? I believe it is also mentioned in the McGee article about the wine wand.
To remove the garlic smell from my fingers, I always rub my hands over the faucet (tap) which is made of stainless steel and that seems to work. Much cheaper than your stainless steel soap gizmo.
Yes, I have also read that plastic wrap can remove some cork flavour. I’ll try to find the article.
e.
Martin: I guess the mechanism is taken from a controlled lab/industrial catalytic process and that it is somewhat hypothetical for the real-life soap application (there isn’t much H2 floating around in the air)? It’s been some time since I read my C-S activation chemistry now, and this is also heterogeneous catalysis which is not exactly my field 😉 Oxygen is of course a diradical, but that’s as far as my guesses go, …if this really works as you mention. According to the comments, it is said to do so.
Lab Cat: I’ll try the faucet this afternoon. Looking forward to it 🙂
thayes: Yes – Harold does mention the plastic trick to remove cork taint. I thought I might prepare a separate post about that where I look at the polarity of 2,4,6-trichloroanisole (the compound giving rise to cork taint) and compare it with some of the flavor compounds.
LabCat: It’s true that you could use whatever stainless stell object you have – even the faucet. But I should mention that the surface of the steel soap is a little rough so you get a certain abrasive effect as well. And in the end the steel soap isn’t that expensive either…
Erik: The H2 was only to regenerate the surface – I believe you could also achieve that with oxygen. Nevertheless I believe the initial steps are a good illustration of how a thiol is trapped and “disarmed”.
Fascinating stuff, I can’t see the wine purists being a fan of these processes though.
Re. the soap – I don’t have any direct experience but we used to sell so many of them when I worked in a kitchen store that they were always on order. People would buy four or five at a time to give away as gifts. Probably our most popular item
Ha, Ha, Ha
Based on the hypothesis put forward for the chemical action of the stainless steel “soap” on mercaptans, I guess that I will have to stop using my stainless steel pans for making garlic-containing sauces. I don’t recall any such disclaimer with my All-Clad pans. Mercaptans are easily oxidized at ambient temperatures, but as far as I know you can’t cleave carbon-sulfur or hydrogen-sulfur bonds under these conditions. Breaking chemical bonds requires energy. This is why catalytic processes are run at temperatures hundreds of degrees above ambient.
If the soap were active in warm water it could not be very selective. It would also attack your skin as readily as it attacks a sulfur bond.
[…] aging of wine? …is being discussed over at khymos.org. Great article, again! February 24th, 2009 | Category: future technology, link, other blogs | […]
are you missing the physical effects here? rubbing a piece of steel against your wet skin is going to strip the surface layers off your skin, presumably the same layers that were just soaking in thiols
kiwi: Good point! I’ve thought about it, but I didn’t mention it in the post. Yes – I certainly think abrasion contributes to the effect of the steel soap!