
Picture by Michael Murphy (CC-BY-SA)
The soda fountain (Diet Coke + Mentos) has been around the net for quite a while with some spectacular videos available, and it has even made it into a news paper cartoon. People go crazy about this and the largest number of simultaneous fountains is steadily increasing.
Despite the interest, only now did a scientific paper appear on the subject. Many have speculated about what causes the reaction between Mentos and Diet Coke, and some have focused on possible acid-base reactions taking place. Mythbusters investigated this in 2006 (watch episode) and came up with the following factors that contribute to the bubble formation:
Diet coke
- carbon dioxide is what makes the bubbles form in the first place
- in synthetic mixtures aspartam, caffeine and potassium benzoate where shown give better fountains
Mentos
- the most important property is the rough surface which provides plenty of nucleation sites for bubble formation
- the density makes them sink which is ideal as the bubbles formed at the bottom of the bottle help expel much more soda
- mentos contains gelatin and gum arabic which could also reduce surface tension
In the paper “Diet Coke and Mentos: What is really behind this physical reaction?” by Tonya Shea Coffey the findings of the Mythbuster teams are largely confirmed.
By measuring contact angles it was shown that aspartame and potassium benzoate reduce the surface tension of water. Aspartame is a winner, and as an extra benefit clean up is much easier with Diet Coke than sugared Coke. The amount of caffeine however is too low to have any effect. The roughness of the Mentos surface was studied with special microscopes (see picture below). Fruit Mentos have smooth patches, but the coating is not uniform and contrary to the Mythbuster experiment normal Mentos and Fruit Mentos performed equally well with regards to foam formation. The roughness of the Mentos surface was inbetween that of rock salt and the Life savers which suggests that roughness is not a single factor determining the reaction. The Mentos surface is covered with gum arabic which reduces surface tension, and experiments showed that even without Mentos, gum arabic could cause a reaction to occur. It is the combined effects of reduced surface tension (due to ingredients in Diet Coke and Mentos) and the rough surface of Mentos which is the key to understand the reaction.
As expected, the article also confirms that the reaction is more vigours at higher temperatures (i.e. solubility of carbon dioxide deacreases with increasing temperature). It was also shown that Mentos sink faster to the bottom of a 2 L bottle compared with rock salt, Wint-O-Green Life savers and sand (this is a function of size and density, not only density). When bubbles are formed at the bottom of the bottle the bubble has more time to grow as it rises. This causes a more explosive reaction and more soda is expelled from the bottle.
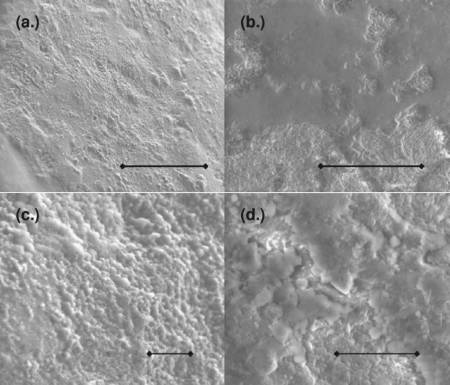
The picture shows scanning electron microscopy images of Mint Mentos (a) and (c) and Fruit Mentos with a candy coating (b) and (d). The scale bars in each image represent the lengths (a) 200 μm, (b) 100 μm, (c) 20 μm, and (d) 20 μm. Fruit Mentos has smooth patches, but the coating is not uniform. (Reprinted with permission from Coffey, T. S, American Journal of Physics, Vol. 76, Issue 6, pp. 551-557, 2008. Copyright 2008, American Association of Physics Teachers)
The question which lingers on my mind is whether Diet Coke and Mentos represent the optimal combination of ingredients to create a soda fountain. With regard to convenience, I guess the answer is yes. But perhaps it’s possible to create an even more powerful reaction? Since lowering the surface tension of water is important, I’m wondering if it would be possible to find a surfactant that could be added without setting the reaction off? Mentos would of course still be needed for the rough surface to provide nucleation sites. In the above mentioned study addition of diluted dish washing liquid was enough to give a pretty good reaction, so this is not an option. But perhaps a couple of drops right on the Mentos surface would work? I definitely need to try this some time.

On the Mythbusters episode they tried rock salt instead of mentos, which worked better (more nucleation sites). I assume that another liquid could be used, maybe with higher concentrations or more efficient surfactants than aspartame, gelatine and gum arabic.
What you will find is that there is an exponential limit that the surfactant will get to before unstimulated loss of gas. The nature of the surfactant will merely affect the concentration required to reach the limit.
A simple experiment (I have done this with 14-18 year old school children) is to add some surfactant (1 drop) then measure the fountain height. Add 2 drops into a fresh bottle, then measure the fountain. Add 4 drops, etc. Plot surfactant versus fountain height and it will look like a Gaussian distribution.
What happens is the change in surfactant changes the width of the distribution, but only slightly effects the height of the fountain. The discovery is that the rate of nucleation is the limiting factor in the system.
Roughly breaking the Mentos or rock salt will increase surface area and hence, rate of nucleation. Powdered material slows the reaction as the top of the solution desorbs gas faster than the bottom, leading to longer but less powerful fountains.
What about letting the soda go completely flat, then adding the surfactant, then re-carbonating the soda? Or would the surfactant prevent the carbon dioxide from dissolving?
Robert: Did you apply the surfactant to the Mentos or add the surfactant directly to the coke? (i.e. perform the experiment without Mentos)
Barzelay: You would be able to dissolve CO2, but this sounds like too much work.
Martin: Surfactant into the soda, recap and agitate by swirling. As the bottle is plastic, an appropriate surface is required for bubble formation, hence, the Mentos or a substitute are required.
I also expect that if you coat the Mentos with surfactant there will not be a large change due to the surfactant not diffusing well but remaining localised around the Mentos. I’m not sure though, so will add it to my notes for the next time we do the experiment.
My friends and I did some experimentation with this reaction for a research project that was recently presented at Brooklyn College Science Research Day. I was wondering if anybody has explored the role of phosphoric acid in diet coke as a surfactant or as a substance that will etch the surface of the mentos, creating a bigger fountaining reaction. Please Reply.