
Among dedicated whisky/whiskey drinkers it is customary to add a little water as this “helps to unlock and release the esters, or flavours, from the fats”. Another site claims that dilution helps “breaking down the ester chains and freeing the volatile aromatics”. Does this make sense from a chemical perspective?
When Erik posted me a question some months ago about why we add water to whisky and the chemistry that is involved, I started to speculate about possible mechanisms and discussed them with Erik. Perhaps the most obvious effect is that the alcohol concentration is lowered. High alcohol concentrations anaesthetises the nose and sears the tongue (as the site metioned above correctly states). This is especially true for cask strength whisky which can exceed 60% ethanol. We considered the possibility of a temperature effect. The obvious effect could be achieved by adding water with a different temperature to either cool or warm the whisky. The less obvious effect could be due to a possible release of heat when adding water to a concentrated ethanol solution. Having thought about the different possibilities I did a search and found a very fascinating article: “Release of distillate flavour compounds in Scotch malt whisky”. It was published in 1999, but was new to me and gave me some totally new perspectives on whisky and water. When reading the article, it seems to me that the motivation for adding water to whisky is in fact to mask some aromas and release others!
Malt whisky contains high concentrations of esters and alcohols with long hydrocarbon chains. When water is added, the solubility of these esters and alcohols decreases, and a supersaturated solution results. In extreme cases, the decreased solubility of fat-soluble, volatile organic compounds can lead to clouding due to precipitation of small droplets as seen with anise/liquorise liqours such as Pastis, Pernod, Arak, Raki, Sambuca, Ouzo… (I think I’ll post about that later some time). This can also occur with whiskys that haven’t been chill-filtered. But even in whisky that has been filtered at low temperature a form of “invisible” clouding will occur. The excess of esters and alcohols in the diluted whisky form aggregates (or micelles) which can incorporate esters, alcohols and aldehydes with shorter hydrocarbon chains. Once these compounds are trapped in the aggregates, surrounded by longer chain esters and alcohols, they smell much less since they have a harder time escaping from the liquid! Fortunately, some of the compounds that are trapped have less desireable aromas described as oily, soapy and grassy.
The presence of wood extracts originating from the aging in oak barrels also influences aroma release. One effect is that wood extracts displace hydrophobic (fat soluble) compounds from the surface layer of the whisky (this effect is significant at room temperature when smelling the whisky, less so at 37 °C in your mouth). Furthermore the presence of wood extracts increases the incorporation of hydrophobic compounds into the agglomerates mentioned above.
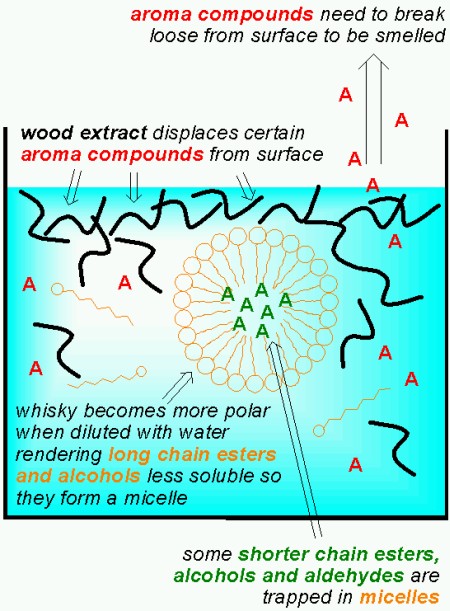
So far I’ve only discussed the aggregates formed by long chain esters. But studies have shown that when an aqueous solution contains more than 20% ethanol, the ethanol molecules aggregate to form micelles, just like the long chain esters do. These micelles can also trap flavour compounds. Unlike the micelles formed by the long chain esters however, the ethanol micelles break up when diluting the whisky, thus releaseing the entrapped flavour compounds. It is interesting to note that ethanol is less “soluble” in water at high temperatures (ie. the solution is no longer monodisperse). As a consequence, serving whisky “on the rocks” will actually promote the release of flavour compounds from the ethanol micelles. As Mirko Junge commented below, this is one of the very few cases where cooling actually enhances flavour! But the wood extracts found in whisky matured in oak casks supports the formation of ethanol micelles, so as Mirko Junge points out, matured whisky needs more dilution and/or cooling since there are more ethanol micelles.
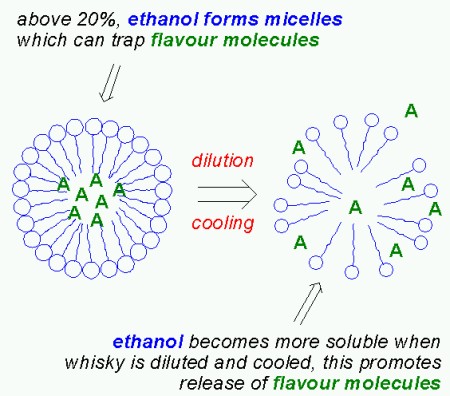
The over-all effect is a fractionation of volatile compounds upon dilution with water: water insoluble compounds are concentrated in the aggregates (or micelles) of long chain esters, water soluble compounds remain in solution and compounds (probably those which are slightly soluble in water) that were originally trapped in ethanol micelles are liberated.
So after all, the popular notion that addition of water “opens up” the aroma of a whisky is true, but who would have thought that the effect is a combination of “masking” (inclusion of some arome compounds in long chain ester micelles) and “demasking” (opening up of ethanol micelles) and that there even is a temperature effect?

Serving whisky “on the rocks” helps break down ethanol micelles due to the combined effect of cooling and dilution. (Photo by Generation X-Ray at flickr.com)
Feel free to share your experiences with whisky dilution in the comments section below!
(Note: The text has been revised and expanded on June 3rd following the discussion below. Special thanks to Mirko Junge for his valuable comments and for pointing out the importance of the ethanol micelles.)

Thanks for this post!
I’ve been following your blog for a while now and it’s time to de-lurk.
To throw more fuel on the fire, I’d like to ask a question regarding Tequila and Grapefruit juice. Recently a friend suggested a drink made from these two things. He described it as ‘tasty’. :-/ I think this could be an interesting study… two things that each onto themselves tastes quite terrible leading to something that isn’t… sounds like chemistry to me.
Further ‘research’ to follow… but this example doesn’t use quite as much of the grapefruit juice as I’m thinking of here…
http://www.extratasty.com/recipe/244/breakfast_margarita
This is really great. So, after reading it (and I think I get it) am I safe to assume that most ‘undesireable’ aromas are non-soluble in water while desireable ones are water soluble… at least in the example of whisky. What other mixtures could be viewed the same way?
Christian,
I doubt science will be able to distinguish between a “quite terrible” taste and the opposite in any near future. Taste preferences are quite individual and to a great extent they’re a result of culture and upbringing. I like grapefruit juice on it’s own for instance… But I’ll take a note of the tequila-grapefruit combination and see if I can find out something.
Chad,
For whisky – as far as I understood it – yes. But since the compounds are not listed I’m not sure about that. And addition of water will probably also mask some desireable aromas as well. Also I think it’s very hard to draw a line between desireable and undesireable aromas (cf. the comment above…) as this will depend on who you ask. I can’t think of any other system now where addition of water results in a masking effect like this.
OK, in essence you propse a theory that says: The water locks away the ‘bad’ taste (molecules) in micelles which are so stable as not to disintegrate in the mouth.
One should device an experiment to test the hypothesis along the following lines: Dilute ‘bad’ tasting whisky until it tastes ‘good’. Separate a fraction of the micelles out of the mixture. The whisky/water mix should still taste ‘good’. Add part of the micelle-‘fraction’ the the whisky/water mix, keep tasting until the micelle-fraction of the mix is well above that of the undiluted whisky. The micelle-‘spiked’ whisky/water mix should taste ‘good’, according to your theory.
I doubt that you will be able to proof you hypothesis. Even though I visited the Jack Daniel Destillery, I am not that much of a whisky-buff, as to try to disproof your hypothesis myself (and I do not have that brilliant an idea to get those micelles out of the whisky/water solution in the first place…).
Mirko,
it’s not my hypothesis or theory. I only try to sum up and communicate the essence of the article “Release of distillate flavour compounds in Scotch malt whisky” (I’ve fixed what seemed to be a broken link to it now). The conclusions in the article were based on a number of experiments performed by the authors.
It may not be a trivial exercise to remove or harvest the micelles from the alcohol. Might involve some centrifugation to separate the two phases.
One could add something to pull the micelles down, precipitate them.
This book, Food Processing Handbook, at the link below may be helpful
http://www3.interscience.wiley.com/cgi-bin/summary/112606273/SUMMARY
Costs $25.00 for the online version.
Martin,
After reading the article (Conner et al.) very carefully, I come to the same conclusion as you (water opens up the flavor in whisky) but the line of reasoning and the underlying effects are totally different to yours. I follow Conner et al very closely:
At higher ethanol levels, the water/ethanol mixture is not as homogenous as one might think (Conner 1999, p1019):
Thus, whisky still in the bottle is a micro-emulsion, which the esters being incorporated into the aggregates. By diluting it with (soda) water the ethanol aggregates disintegrate and the esters, i.e. the flavors, are released.
The temperature dimension (Conner 1999, p.1019):
Thus, there is a case for whisky on ice: The ice cubes cool down the whisky and bring the “˜stuff’ below the point of aggregation with a consequent release of the “˜flavors’ from the dissolving aggregates! One of the rare instances of more flavors by cooling the “˜food’.
Adding the maturing in oak casks (Conner 1999, p 1020):
Thus, their need to be more dilution and/or cooling in wood cask matured whisky.
Furthermore (Conner 1999,1019):
As well as an annotated quote from the Conclusions:
The maturing in wooden casks results in a greater capacity of the ethanol/water-mixture to incorporate shorter esters (bigger ethanol micelles), reduces the evaporation of the shorter esters (the longer esters are covering the fluid-air interface).
Do you have any idea, how these lines of reasoning (resulting from the same paper) could be made to converge?
A very thorough comment there Mirko! You make a very good point about temperature and the fact that for a whisky “on the rocks”, the lower vapor pressure due to cooling is counteracted by the fact that the ethanol aggregates break up. I missed that point!
As you can see from the original post, I didn’t mention ethanol aggregates at all, only the aggregates formed by the long chain esters upon dilution which are mentioned in the introduction. But thank you for discussing the importance of the ethanol aggregates! I certainly oversimplified matters a lot – which is probably noticeable from the fact that your comment is as long as the original blogpost 😉 But thanks again for bringing additional aspects to my (and other reader’s) attention!
Sorry… this is an unrelated topic. Just wasn’t sure how else to contact you. Also not sure if this news piece is circling the globe through other means, but it’s well written and thought I’d forward it.
http://www.cbc.ca/news/background/senses/umami.html
[…] and Waitresses must be familiar with the science of food and drink (and should be tipped […]
as per the tequila and graperfruit… i have a mexicano friend who told me that one of his favorite drinks is tequila y toronja( naturally flavored grapefruit soda) it is delicious on its own as well as combined…. we get a case of the soda from our local restaurant depot and a few bottles of tequila for cookouts. its always a hit.
as far as the whiskey information goes…. i recently had a scotch tasting @ a restaurant i was working at, i was not a big scotch fan @ this time i prefered tennesee and irish for their lack of peat flavor & aroma… then i was instructed to dilute each of the scotch whiskeys as well as taste them straight up. the change in flavor was tremendous. its interesting to get the actual chemistry on why this happens. im sure this was discussed in the material but, would scotch be affected more by dilution or cooling vs other whiskeys (sans peat) due to the oils from peat smoke? i prefer my whiskeys w/ a little cold water, vs ice… i find when its too cold it has less flavor, but according to the info i might be wrong…. is there a tempature barrier, too cold? whats a good dilution ratio?
nerdy,
I think the lenght of maturation would be more important – i.e. older whiskies would need more cooling/dilution.
For nosing the whisky I would recommend cold water. For drinking however, ice-cubes would probably be better since more cooling would release more flavour. Once in the mouth, the temperature would rise to 37 °C anyway. But there is a trade-off, since since cooling will release flavour from the ethanol micelles, but lower the vapour pressure (i.e. the whisky will smell less).
[…] of water, the ‘molecular gastronomy’ blog, khymos, offers an interesting perspective on the old question of whether water releases the aroma of whiskey. Once again, and not surprisingly, chemistry is involved. Must read for geeky scotch […]
does alcohol level play any role to this discussion? one of the reasons to dilute whisky is to lower the alcohol level and control for the numbing effect it might have on the palate and olfactory system. since the more a whisky is matured, the lower its alcohol content is (due to evaporation during maturing), older whisky’s would have less alcohol, hence would need to be diluted less. what is your take on this?
Redrum:
Yes. As I wrote: “high alcohol concentrations anaesthetises the nose and sears the tongue”.
Although maturation might influence the alcohol level, many whiskies are typically diluted before botteling. So the alcohol concentration of the end product is not really influenced by ageing. One effect of maturation is that more compounds are extracted from the wood. These compounds influence the aroma both directly and indirectly (by formation of micelles and trapping). Therefore it would be a good idea to dilute older whiskies as well as younger ones to “unlock” the aroma.
as an avid single malt scotch whisky enthusiast, i have for several years now been explaining to friends, family and strangers the necessity of adding just a few drops of spring water to open up the aromas and flavors of a quality scotch whisky. (as a disclaimer, i have a small list of scotches that remain indifferent to this technique, and a few others that actually respond downright poorly…)
in any case, a while back, a friend of mine sent me a link for a curious product: polished granite cubes from Scotland meant to be chilled and placed in a dram of whisky to cool it, like ice. naturally, we mocked this product greatly, not least for the anticipated damage “granite cubes” would do to our Reidel single malt glasses, but mostly because, well… real men don’t chill their scotch.
however, having read your excellent blog post and the referenced article, i guess i can actually see a use for a product that could chill a dram below the point of aggregation, without at the same time diluting it beyond the number of drops needed to achieve the whisky’s maximum flavor profile.
i am in no way affiliated with the company or product, and despite what i’ve said have no plans for a purchase. however, just because it fits the discussion so well, i will provide a link here:
http://www.sippinontherocks.com/
thanks to all contributors for a thought-provoking read…
[…] cubes are used both to cool drinks, but also to significantly impact the flavour of certain drinks. No matter your motivation, you should never use “old” ice cubes […]
RSC has an article on whisky which also touches in on the subjects I discussed in this post:
http://www.rsc.org/chemistryworld/Issues/2008/December/AWhiskyTour.asp
It’s well worth reading!