
Lussekatt (Photo by Jonas Bergsten)
The day of St. Lucia is celebrated in Scandinavia and some countries in southern Europe on December 13th. In Scandinavia a traditional kind of bun, lussekatt, is normally made and eaten on this day.
What is exciting about this from a chemical perspective is that they are made with saffron, the world’s most expensive spice (a recipe can be found here). Because of the high price, saffron is sometimes adulterated with turmeric. There is however a simple chemical test to check whether your saffron has been adulterated or not.
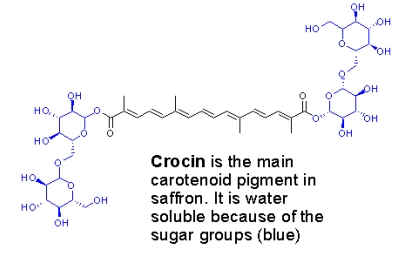
The color of saffron comes mainly from crocin, a carotenoid with a sugar attached that makes it water soluble (this is why the color is so easily extracted into water containing foods):
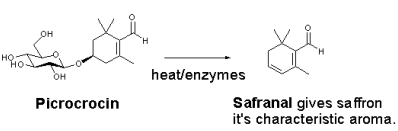
The aroma arises mainly from the degradation of picrocrocin to release the terpene safranal:
The yellow color of turmeric comes from curcumin.
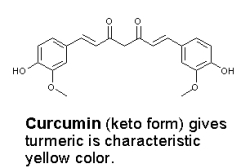
Upon reaction with a base, curcumin turns bright red whereas crocin is unchanged. Because of this it should be possible to detect whether saffron has been adulterated with turmeric. In the picture below, strips of coffe filters where inserted into suspensions of saffron and turmeric in water (two of each), and those on the right where then held over a bottle of aqueous ammonia. An immediate reaction takes place between ammonia and curcumin, producing a bright red color. I should quickly admit that I haven’t had the opportunity to test this on an “authentic” adulterated sample!
BTW, the color change is very fast as is obvious from the video below (click here if it doesn’t play in the window below):


[…] Lersch di Khymos controlla la genuinití dello zafferano con i vapori di […]