My fellow blogger on molecular gastronomy, Göde Schüler (check out his German MG blog Gourmetrics) found a great video on YouTube. The video shows how a red beet paste mixed with alginate solidifies when dripped into a solution of calcium lactate (this solution is normally clear, the yellow colour comes from extensive use).
Chef Simon (French, click here for babelfish translation) has a nice page on alginates as well. Another french page here (with english translation by babelfish). You can find links to more technical information (free pdf’s) on alginates in the static pages of khymos.org.
The chemical principles put simply are as follows:
Sodium alginate is water soluble and can be mixed with many different fruit/vegetable juices and purés. When dripped into a solution containing calcium ions, each calcium ion (which holds a charge of +2) knocks away two sodium ions (each holding a charge of +1). The alginate molecule contains loads of hydroxyl groups (OH’s) that can be coordinated to cations (that’s ions with a positive charge such as sodium and calcium).
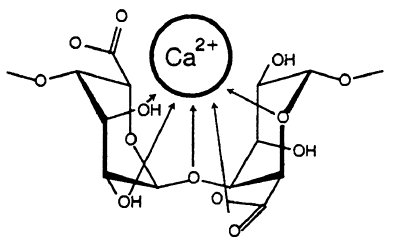
Figure from Draget, K. I.; Smidsrød, O.; Skjåk-Bræk, G. “Alginates from Algae” in “Polysaccharides and Polyamides in the Food Industry. Properties, Production, and Patents”, Steinbüchel and Rhee (Ed.), Wiley 2005.
When alginate is coordinated to sodium, it’s a very flexible chain. When sodium is replaced by calcium however, each calcium ion (black dots in the image below) coordinates to two alginate chains, linking them together. The flexible chains become less flexible and form a huge network – a gel. The fun thing is that this happens within seconds after the alginate mixture is dripped into the water bath with the calcium ions.
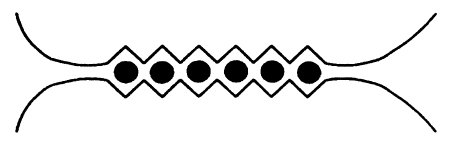
Figure from Draget, K. I.; Smidsrød, O.; Skjåk-Bræk, G. “Alginates from Algae” in “Polysaccharides and Polyamides in the Food Industry. Properties, Production, and Patents”, Steinbüchel and Rhee (Ed.), Wiley 2005.
Approximate concentrations:
- Fruit/vegetalbe juice/puré with 1-2% sodium alginte
- 2% calcium chloride solution (approx. 10g in 1/2 L of water) – because calcium chloride has a slightly bitter taste, it is a good idea to rince these pearls with water before consumption. This is also the reason why calcium lactate is often used in stead (as shown in the video).
Update: The Frog Blog has nice posts with pictures showing how Jay Veregge and Joel Robuchon utilize alginate gels. Also, check out this “caviar” maker for dripping 96 drops of sodium alginate solutions into calcium chloride at once.

Just wanted to drop you a line and tell you that I appreciate the link to my page. I really enjoy what your doing a the the kymos blog and visit your page often in hopes of cool new posts or to use a previous read post as reference. Drop me an e-mail so that we can easily share ideas and research topics in the pursuit of our common goal; learning about and creating the coolest food possible.
Thanks again,
Chef Frog
please send itto me i wan’t to learn more about it
[…] Here’s some pictures and a video of my first experiments with sodium alginate and spherification. I used sodium alginate from the Texturas series and calcium chloride from a drug store. Needless to say, I’m very fascinated by the texture and the whole process. I have blogged about the chemistry behind previously. […]