
Last year, while visiting family in Germany, I decided to pick some walnuts to bring home to Norway. They were not ripe, which was good, because I was planning to make nocino, a walnut liqueur. You can easily find a number of recipes by googling and there is also a nocino-thread over at eGullet.
What fascinated me the first time a saw nocino mentioned in a book about liqueurs was the nearly black color. Many recipes comment that after steeping, the liquid looks more like used motor oil than something edible. The color is really amazing and I also observed that most recipes recommended the use of gloves as the stains from the unripe walnuts would not easily come off. The juice from the walnuts is a light yellow green color to start with, but when exposed to air it quickly turns dark brown. Color chemistry is always fascinating and I couldn’t resist the temptation to investigate this further.

This picture was taken only 3 days after I steeped the walnuts in ethanol.
I did a little research and found a number of scientific papers about nocino. Some of them focus on the high content of phenolic compounds and possible health effects. Personally I can’t really see how nocino will ever become a significant source of phenolics in anyones diet. Even the article entitled “Influence of processing variables on some characteristics of nocino liqueur” is mainly concerned with how the process variables affect the antioxidant activity. There is no mention of taste or aroma (and this is perhaps why I would not classify these papers as molecular gastronomy), but the authors conlcude that walnuts picked earlier (more unripe) combined with a long steeping time (3 months) give a higher content of phenolics. They correlate the phenolic content to a measure of firmness, but for those making nocino at home an easy way to test the walnuts is to pierce them with a knitting pin – the walnut should be soft all the way through. As a side note I should mention that the walnuts I used were picked in August which is way too late – the inner walnut shell was stone hard, but I decided to give it a try anyway (more on how it all worked out in a comming follow-up post). The closest I came to some input regarding aroma was an article were 12 different phenolic compounds were analyzed in walnut extracts made with 40, 60 and 96% ethanol. Although the total phenolic content was highest when using 96% ethanol, they found that the concentration of some phenolics (protocatechuic, sinapic and p-coumaric acid) increased with the concentration of the ethanol used for extraction, whereas other phenolics (gallic, chlorogenic, vanillic and syringic acid, (+)-catechin, juglone) were best extracted with 40% ethanol. Polyphenolic compounds are normally bitter or astringent. The low molecular weight compounds are typically more bitter. With increasing molecular weight bitterness decreases whereas astringency generally increases. The solubility in water decreases with higher molecular weight. As I mentioned in a previous post on ethanol extractions this is the reason why 30-60% ethanol is most commonly used for infusions and extractions.

Even though the contents of the jar seems to be black, it’s actually still a bright green color if you shine enough light through it. The reason for this is that the jar was kept well closed. Once the nocino is filtered and thereby exposed to plenty of air the color will change to dark orange brown.
Despite all the papers on nocino I still didn’t know more about the colors of nocino, and what I was really searching for was an account of the color changes I observed. The incredible staining of skin and the change from bright green to dark brown and nearly black upon contact with air. Back in 1856 Reischauer and Vogel [1] studied and isolated a compound they named juglone after the latin name of walnut (juglans). In 1885 the structure was elucidated by Bernthsen and Semper who two years later also synthesized the compound [2]. Later Mylius [3] isolated a precursor to juglone, α-hydrojuglone, which upon oxidation yields juglone. And in 1950 Daglish [4] showed that what had become known as “apparent vitamin C” was in fact a hydrojuglone glucoside, a precursor to α-hydrojuglone. This is a nice illustration of how the most stable molecules are discovered first, before one realizes that there are more reactive precursors.
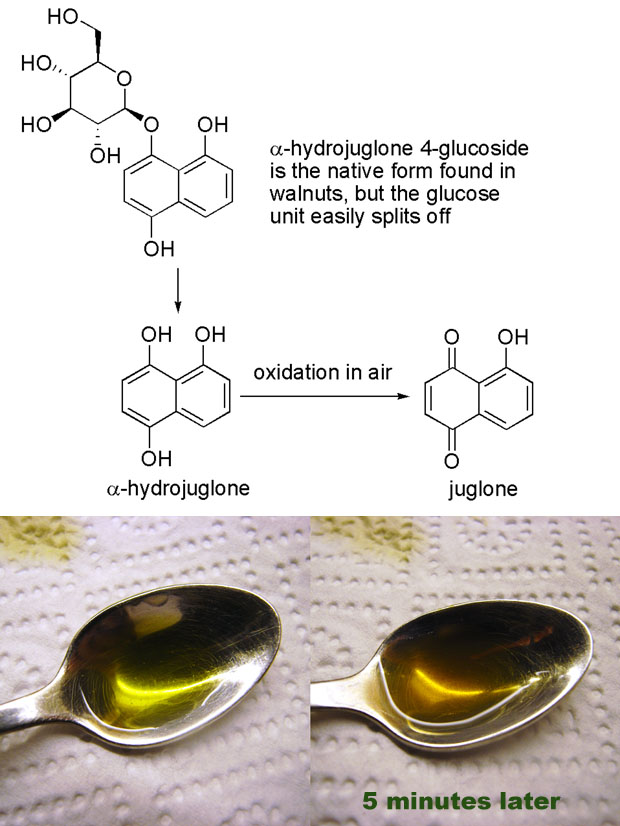
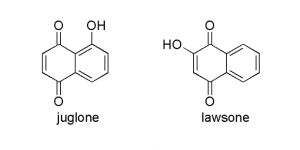 It’s also interesting to note that the structure of juglone is closely related to lawsone, a compound found in the henna plant (Lawsonia inermis) which is used for skin coloring. The hydroxyl group on the quinoid double bond causes lawsone to easily undergo oxidative dimerization (which I belive leads to compounds with a darker color – this would explain why henna color typically needs some time to develop). In the henna plant lawsone exists as a glucoside, and I would suspect that it is in the form of hydrolawsone glucoside which looses glucose and is oxidized as is the case with hydrojuglone.
It’s also interesting to note that the structure of juglone is closely related to lawsone, a compound found in the henna plant (Lawsonia inermis) which is used for skin coloring. The hydroxyl group on the quinoid double bond causes lawsone to easily undergo oxidative dimerization (which I belive leads to compounds with a darker color – this would explain why henna color typically needs some time to develop). In the henna plant lawsone exists as a glucoside, and I would suspect that it is in the form of hydrolawsone glucoside which looses glucose and is oxidized as is the case with hydrojuglone.
Read on: Nocino – walnut liqueur (part II) – includes recipes!
References:
[1] Vogel, Reischauer Jahresberichte 1856, 693.
[2] Bernthsen, Semper Berichte der Deutschen Chemischen Gesellschaft, 1885, 18, 203. Bernthsen, Semper Berichte der Deutschen Chemischen Gesellschaft, 1887, 20, 934.
[3] Mylius, F. Berichte der Deutschen Chemischen Gesellschaft 1885, 17, 2411. Mylius, F. Berichte der Deutschen Chemischen Gesellschaft 1885, 18, 463.
[4] Daglish C. Biochem J. 1950, 47, 452. Daglish C. Biochem J. 1950, 47, 458. Daglish C. Biochem J. 1950, 47, 462.

Nocino is on my to-do list this year. In the US no one has availability til around June.
I don’t comment often. I’m a chronic lurker after all. But, you have some of the greatest blog posts. Please, keep up the good work.
I was incredibly interested by your comment that, “In general phenolic compounds are bitter and astringent, and the bitterness generally increases with increasing molecular weight.”
It has been my understanding that lower molecular weight penolic compounds are bitter and as they increase in molecular weight they become less bitter and more astringent until finally at a certain molecular weight they are virtually insoluble in water, and hence neither bitter nor astringent.
Looking through my notes, I found only this little bit:
“Food Flavors: Biology and Chemistry” by Fisher notes that monomeric polyphenols are more bitter than astringent, and that as they complex to form larger polymers, they become more astringent and less bitter.
Unfortunately I don’t have the book available to me to confirm. Do you have any further information which would clarify things?
Best,
Alan
P.S. I love your blog.
Martin: Very nice written post, your passion for the matter really shines through. Looking forward to part 2.
Alan: You are absolutely right – thanks for reminding me! I’ve changed the sentence now to read:
And if we were talking about wine it would be the medium and high molecular weight compounds that give wine it’s “body”.
And to the rest: Thank you so much for your kind comments 🙂
I’m just ordering my walnuts now for this; they’ll be picked next month. Looking forward to your part 2 for some tips…
Hi again; fascinating post!
The oxidative dimerization dimension reminds me of tea processing: after wilting to reduce moisture content, the leaves are (may be) bruised to rupture the cell walls, releasing polyphenol oxidase enzymes (and leaf juices). The green/yellow catechins polymerize into orange/red theaflavins and thearubigins, till the enzymes are inactivated by heat.
(There was a post to rec.food.drink.tea a few years ago about why some Darjeeling teas appear greenish despite black tea processing: http://groups.google.com/group/rec.food.drink.tea/msg/2f8cdd1418a3be84?hl=en )
I wonder to what extent is nocino’s oxidation enzyme catalyzed. If you, say, heat and cool the green nocino before it turns brown, would it stay green longer? I wonder how it’d taste
Naterejan:
I”m hoping you could help correct or explain why my recent batch of Nocino has a medicene taste to it. I believe in the adding of the sugar we used a sugar normally used for making of candy instead of the regular granulated we have used in the past. Our second thought is that perhqaps it is a fault of the walnuts themeselves which were smaller than noemal.
regards,
gabestogie@hotmail.com