
Brazilian chiles in oil (very nice with Moqueca!)
Oils and fats are long molecules which are mainly non-polar and hence the opposite of water which is a polar molecule. Ethanol which has both a polar and a non-polar end falls in between oil and water. I’ve covered extractions using water and ethanol previously. That water and oil are opposites is easily observed by the fact that they don’t mix, and because of it’s lower density oil floats on top of water. This property allows us to easily separate water and oil.
Volatile molecules – the molecules that we detect by their smell – are mainly non-polar and therefore soluble in oil. This is one reason why foods with fat often have a different and often better flavor compared with their fat-free counterparts (fat of course also influences mouth feel etc.). Everytime you cook with oil it will actually help extract aroma (or smell flavorants) from the food ingredients and deliver these to your nose.
There are several oil extracts used in the kitchen, and the nice thing about them is that the oil extracts aromas and then protects them from the air. This is good as it prevents oxidation of the aroma molecules, but in some extreme cases bad because the anaerobic conditions may promote growth of botulinum spores – more on that in the last paragraph. When the flavored oil is added to a dish you get can immediately perceive the aroma. If the oil is tasted pure it serves as a carrier for the aroma giving a small explosion in the mouth (or nose to be more precise…). Some examples I can think of where the oil plays an important role in extracting and delivering aromas are: pesto, tapenade, mayonaise, aioli, curry paste (and all other spice pastes), chili oil and truffle oil to mention a few. Notice that in most of these the source of the aromas is still present in the oil.
One significant addition to the aroma molecules is capsaicin which gives chiles their pungency. Capsaicin is not particularily volatile so it never reaches your nose, but it certainly does burn your tongue! The funny thing is that the receptor being attacked by capsaicin is a protein which is also sensitive to temperature. So when talking about “hot” food it’s true in a double sense. There is an overlap in how our brain perceives food which has a high temperature and food which is spicy.
The fact that water and oil are non-miscible can be utilized in the kitchen. Oil can be used to extract non-polar compounds from a water phase, and oppositely water can be used to extract polar compounds from an oil phase. In the organic chemistry lab water and oil would be separated with a separatory funnel, but in the kitchen a normal plastic bag will work fine. Check out the pictures and description of how a plastic bag is used to clarify butter over at Cooking for Engineers.
Although most of the aroma molecules will be present in the oil, a tiny amount will remain in the water. It is possible to measure how molecules partition between oil and water, and instead of cooking oil one uses octanol. You can read more about the partition coefficient Koctanol/water over at Cumbrian food lab.
To start experimenting with this in the kitchen I suggest you start with some colored foods. Flavor compounds are normally colorless so it’s hard to see where they end up. One can put up a very general list of compunds responsible for the color of foods:
We can start with blueberries. For the experiment I used a blueberry syrup and mixed it vigorously with oil using an immersion blender. However, when the phases separated the oil was colorless and the waterphase was still blue. The reason for this is that anthocyanins which give blueberries their nice color are water soluble. No matter how much you blend the blueberries with oil the blue color will remain in the water phase.
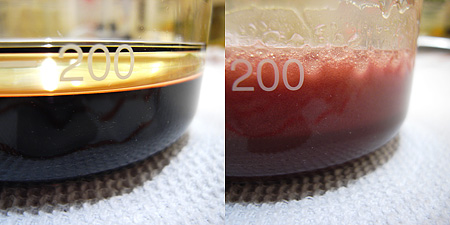
I should have waited longer to allow the phases to separate properly, but notice the oil clinging to the glass wall in the right picture – it’s totally clear without any traces of blue/purple color.
For our next experiment we will use carrots or carrot juice. Add some oil and mix with an immersion blender to extract the carotene. What you observe now is that the oil phase turns orange/yellow. The reason for this is that the carotenes are oil soluble. If desired one can separate the two phases with a plastic bag as mentioned above.

Extraction of carotene from carrots. Pictures: 1) I finely grated carrots, 2) Blended them with water and filtered of the remains – the water phase was then layered with plain cooking oil 3) Water and oil were mixed with an immersion blender and the phases left to separate, 4) A plastic bag serves as a separatory funnel – cut a small hole to let out the liquid. The water phase turned grey, probably because I left it at room temperature to allow the phases to separate (1-2 days).
Now that the effect has been demonstrated with food colors it’s time to move on to tastes and aromas. The four basic tastes are all soluble in water, whereas the pungency found in chiles for instance is soluble in oil. Aromas or smell flavorants however are primarily soluble in oil. To test this one can take some clear meat stock, add oil and taste the water and the oil phases separately. The water phase will be salty, and also have a little meaty flavor (our nose detects the tiny amounts of oil which remain in the water water phase, even if no oil droplets can be seen – and of course there are also umami flavorants in the water phase). The oil phase will not be salt at all and have a strong meaty aroma.
Even though you seldom will go to the extremes of separating oil and water phases, it can be good to think about where your aromas goes when you cook. And so you won’t forget I rewrote the first few lines of the Shoop Shoop song:
/ D7 – C7 – / D7 – – – /
Can you tell me where the aroma goes
and how it enters into my nose?
/ Am7 D7 Am7 D7 / / G Em7 Am7 D7 / G C D – /
It’s through the oily phase – Oh yeah, into the nose
In the water phase? – Oh, no, that’s just the salts
If you wanna know where the aroma goes
It’s in the grease, that’s where it is
(aroma should be pronounced more like ‘roma when singing)
—
Somes words about safety: When infusing spices, herbs or garlic – think about the fact that you create anaerobic conditions. If pH is above 4.6, the oil is kept at room temperature, and Clostridium botulinum spores are present you might be bad off (botulinum toxin causes botulism). There are sites that cover this in greater detail. Perhaps the easiest way of preventing the growth of botulimum spores is by adjusting the pH with an acid such as phosphoric or citric acid (that would be the pH of any water phase present as they are not soluble in the oil).

Hi Martin,
thanks for the nice post 🙂 I have got a couple of question:
What about infusing dried spices? no worries about Botulinum or am I wrong?
What is the effect of temperature? I know that it should help the liberation of aromatic molecules but what about the oil itself? Heat changes the oil tastes… Which is the best oil to use then?
Thanks a lot 🙂
During a little (absolutely non scientific) experiment I extraced lemon peel flavor into oil in less than half an hour, simply by heating the oil to a safe-ish 110 degrees centigrade, so that seems to suggest heat helps speed the extraction (for comparison, try dumping lemon peel in cold oil – it will take days to extract flavor.
but does heating up “break” down the flavour or change it. they say if you heat cocoa over 90C the flavours start to disappear and not become as rich.
I guess both temperatures (extraction and damage) depends on the odorant profile we want to recover. The heaviest module efficiently contributing to the wished aroma might have the higher release temperature (so pushing us to rise the oil temperature) while instead the other molecules already released could start to be deteriorated.
Could it be possible to reconstruct something closer to the original profile using parallel extractions (i.e. same material, different batches) at different oild temperature? (kinda playing as parfumier :p)
What would be interesting was to see how chlorophyll distributes between water and oil, and whether the colour in i.e. cooking water is purely a chlorophyll derivative or chlorophyll itself (I did some informal kitchen experiments on this):
Chlorophyll (or it’s derivative compound) colour is pH dependent (in addition to the anthocyanins). Add some acid, i.e. vinegar, to the green cooking water and the colour vanishes; add some base (bicarbonate, cleaning ammonium chloride) and the colour returns.
Alessio: To be honest – I don’t know. But using oil to extract dried spices seems like a big compromise to me. Temperature will speed up extraction, but can also affect the aroma molecules. I wouldn’t be too concerned about the oil regarding temperatures up to 100 C. Regarding choice of oil – chose one with a neutral taste.
Erik: Cool experiment and video!
Erik: chlorophyll is similar to a surfactant, in that it has a long non-polar chain attached to a polar headgroup (think of a ball attached to a rope). The ball has 4 sites that can bind to a metal centre – Magnesium mostly.
When attached to the metal, chlorophyll is green. However, at low pH the 4 sites become protonated and the metal is released into solution, leaving the colourless chlorophyll. Neutralise the acid and the metal becomes rebound.
Chlorophyll will become more soluble in non-polar solvents at low pH as the headgroup becomes less polar when it is protonated. The non-polar chain is too big for the molecule to become readily soluble in water. At high pH the molecule will act as a reverse surfactant and allow water to be miscible with oil.
I usually do tomato confit with chili, and garlic that is roasted in the oven. This leaves lovely tomato flesh and an roasted garlic and chili oil you can filter and separate. This dish doesn’t lose anything by heating IMHO. It contains an amazing flavor. And I keep it in the fridge afterward. So if you want to try this idea and want to play it safe this is a good start. Someday I’ll try Heston’s idea of infusing the tomato wine somehow.
Can be served as a starter, on canapes, as a pasta dish, and so on.
[…] The food pairing I found most interesting was the one with Oud Brugge (a cheese), coffee and vanilla. To bind these flavors together chef Gert de Mangeleer from Hertog Jan used potatoes. The surprising element of the dish was the coffee – he sprinkled his dish with freshly ground coffee. The vanilla was applied as a grape seed oil extract of natural vanilla – a nice example of how oil can be used for flavor extraction. […]
I’m obviously very late to the conversation, but I found this method for making vanilla oil and extract together. http://lists.whatwg.org/pipermail/herbalist-ansteorra.org/2000-November/005019.html
I wonder if shaking up the mixture of oil and alcohol is a reliable way of preventing the growth of botulimum spores?