
Photo by Mel B via flickr.com (CC).
Measuring powders by volume has serious limitations (more on this later in an up-coming post), but one great advantage is that for small quantities going by volume can sometimes be more accurate than weighing them. At least when you work in a kitchen and don’t have access to professional lab scales. When a scale shows 0.1 g, the true weight could be anywere from 0.05-0.149 g due to rounding (that’s ± 50%!). Not to mention the fact that cheap balances aren’t always very accurate for such small amounts, even though they feature a 0.1 g resolution.
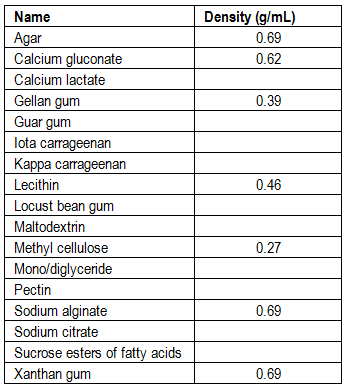
I’m currently working on a major revision of the collection of hydrocolloid recipes. One thing I would like to include is a table with densities of the hydrocolloids and chemicals used. When the densities are known, it’s possible to give some rough advice for what volume to use (this on-line conversion calculator has the densities of many common ingredients). This could ease small scale preparations. It will also make it easier to calculate the percentage of hydrocolloid used in recipes where the amount is given by volume. I’ve measured the hydrocolloids I have at hand, but I need your help to fill out the table and repeat the measurements I’ve done. With enough measurements I could also do some statistics and make a plot. I’m also interested to see if there is much variation between different brands.
How to determine the density:
- Find a suitable measuring spoon, cup, shot glass, container – whatever you have – with a volume of at least 10 mL (I used one of about 30 mL).
- Put the empty container on the balance and use the tara function.
- Fill completely with water and weigh again. The difference gives you the exact volume (for water 1 g = 1 mL).
- Dry the container, put it on the balance and use the tara function.
- Spoon the hydrocolloid into the container, tap the side gently once or twice with the spoon and level off.
- Weigh the container again and write down the mass of the hydrocolloid.
- To calculate the density of the hydrocolloid, divide the mass by the volume you obtained for your container. This gives you the density of the hydrocolloid with units g/mL.
Repeat steps 4-7 for each hydrocolloid you have at hand. I would very much appreciate if you email your results directly to me at webmaster (@) khymos (.) org. Please include the volume you measured (larger volume means more accurate measurement) and which brand you used. It will be interesting to see if the brands differ a lot.
I should add one coment about the products from texturePro: this picture indicates that all (?!!) the texturePro hydrocolloids are mixed with maltodextrin (please correct me if I’m wrong – it could be that this only applies to the cocktailPro kit). And I think the same is the case for several of the Sosa products. This increases the volume and eases the use of a measuring spoon (which comes with every texturePro kit), but unless the exact proportion of hydrocolloid to maltodextrin is known, following other recipes than the onces included with the kit is more or less impossible. Let me know if you have further details on the hydrocolloid/maltodextrin ratio in texturePro or Sosa products.
In advance: Thank you very much for your help!

High resolution (.002 gm) digital scales are now inexpensive ($65).
http://preview.tinyurl.com/6kgo95
Medium resolution scales (.01 gm) are even cheaper ($45).
http://www.canadianweigh.com/scales/mxt-series-scales
Volume measurements always have the problem of how much the powder has packed down. One must first sift the powder then extract the needed volume. I once bought several teaspoons made by different manufactures and the variation in their volume was enormous when I tested them measuring table salt and a high resolution digital scale.
There are several problems with measuring density of solid materials.
Firstly, packing and air bubbles. Just pressing down firmly is not going to be accurate enough, and how do you do so consistently? Tapping can help, but in a reproducible way?
Second, contaminents. Water, salts, buffers can all dilute the solid and alter mixing. You would need to determine values from different sources.
Third, you did not mention repeating the experiment. You should take at least 3, preferably 5, measurements. Weigh, empty and refill container, reweigh.
Yes – I certainly agree with you! The best way to measure is by weighing accurately. And density measurements of powders is difficult and has poor reproducibility.
But as I stated in the post, there are a couple of reasons why I still want to record (approximate) densities:
1. Several recipes I find state the amount of hydrocolloid used in teaspoons or tablespoons (about 5 and 15 mL respectively). In order to calculate the percentage used I need to know an approximate density.
2. For some kitchen applications (perhaps more in a home kitchen than in a restaurant kitchen) it would be nice just to have a rough idea of the density. I store my accurate balance in it’s original box to protect it from shocks, and some times it would be nice to be able to add, lets say 0.25 mL of xanthan with a measuring spoon and know approximately how much I actually added.
3. It would also be nice to know to what extent the particle size and brand will influence the density.
Unfortunately there hasn’t been much response to my call for help, but I hope some of you can take the time to do some measurements 🙂
I have plenty of hydrocolloids and I’d love to help you but I don’t want to spin my wheels. Can you figure out some sort of shared document or update the post to show what you already have and what you may need? The other barrier is having to deal with fine powder/dust. Moving that amount to and from containers/bags is just going to be messy.
I decided to try a different method, because I think that with smaller amounts of hydrocolloids, people will use smaller measures than 10ml.
NH pectin, LM104AS
I first tried a “teaspoon”, which claimed to be 5 ml. It held 4.68 gm of water when the water was as close to flat as I could make it, or 5 gm even when the water was beaded up over the top (totally full).
I dipped it into my bag of pectin and packed against the side of the bag until flat, and got the following measures:
3.53
3.66
3.75
I also tried dipping and tapping until approximately flat
3.33
3.25
3.29
And dipping, and leveling with a straight-edge
3.03
2.99
3.23 (perhaps this one was more compacted to start?)
I also tried with a “1/4 tsp” measure, which held 1.25 ml of water when as flat as I could get it. I only tried the dip-and-mash method:
0.95
1.00
0.91
novalis: Thanks – using the dipping+tapping and dipping+leveling data (which are closest to what I did), I get an average density of 0.68 g/mL for pectin.
Aren’t starch solutions hydrocolloids as well? I am asking from a purely practical reason, because I am having troubles with my fruit pies being too runny. Even a tablespoon of corn or tapioca starch doesn’t help
Yes they are. Are you using a full size measuring spoon holding 15 mL? Do you follow the instructions for dispersion and hydration?
Yes, I use a full 15ml tablespoon. The directions basically say to stir the starch into the fruit/sugar mixture and pour it into the prepared pastry crust.
Thanks for your reply.
With normal cornstarch you have to heat the mixture for it to thicken.
I have an at least tenuously-related question, if anyone feels like helping a brother out. I want to dissolve a teaspoon of powdered spirulina (basically, dessicated blue-green algae) in a glass of apple juice. The powder has a terrific tendency to clump. If you agitate the mixture, it froths, which is bad. Is there some type of neutral emulsifying substance I can add to this to make the powder dissolve evenly?
Use an immersion blender. This will incorporate air, so do this the day before you need the mixture so that the bubbles can escape. Alternatively you can grind the powdered spirulina with approximately 3-5x the amount of sugar. This will also help dispersion.
Martin: I got a KitchenAid immersion blender (“Empire Red” in color I’ll have you know) and it works perfectly for this purpose. It doesn’t aerate the mixture nearly as much as I had feared – the concoction is ready to consume immediately. I think I’m gonna be having a lot of smoothies this summer!
Cheers mate, thanks for the help –
Martin,
A German book « Avant-Garde molekularküche und andere progressive kochtechniken » (édition Fackelträger) mentions a conversion table between Texturas ingredients and TexturePro ones. The table is available in this French forum link http://fr.molecularcuisine.org/forum/showthread.php?tid=20.
Nevertheless I translated it for you below:
Texturas—g per 100 ml—-TexturePro —–dose TexturePro per 100 ml
Algin————2g—————-Algizoon————— 1,5 single spoon
Calcic———–1g—————-Calazoon—————3
Agar————1,6g————-Agarzoon—————-2,5
Xantana——–1g—————-Xantazoon————–2
Gelan———–2g—————-Gelazoon ————– 4
Iota————-0,6g————-Iotazoon—————–2,5
Lecite———–0,5g————-Emulzoon—————-3,5
Metil————3g—————Celluzon——————7
Stan: This is brilliant! Thanks!
Martin,
Please find below Molecularcuisine.org ‘s contribution to your project consisting in collecting information about ingredients density. To summarize this post in French we gathered the information already available on Kalys’ site and a table published in Anne Cazor French book called “Petit précis de cuisine moléculaire”.
One major manufacturer is now missing to this list: Texturas (TexturePro is of no interest for the purpose of your table and SOSA products are sometimes “technical” products mixed with other substances).
Cheers
http://fr.molecularcuisine.org/forum/showthread.php?tid=139
Thanks! I’ll include it in the next update.
[…] Figuring out food science: A call for help on hydrocolloid chemistry. Khymos […]
[…] of course also depends a lot on the density of the coffee – and here we’re back to why volume measurements are quite useless for dosing powders. After some experimentation however I’ve ended up with a dose around 15-20 g (ground at […]