
Ice cubes are used both to cool drinks, but also to significantly impact the flavour of certain drinks. No matter your motivation, you should never use “old” ice cubes which have been sitting in your freezer for a while. Why? Melt some “old” ice cubes and taste the water. You’ll smell why! The reason is that volatile compounds in your freezer slowly find their way into the ice cubes which for some reason mostly are made in trays without a cover. But as I surfed around, researching this post I discovered that oxo and other producers now sell ice cube trays with lids. That’s a small step forward!
Another thing about ice cubes is that they look nice. I admit that air bubbles can sometimes be quite beautiful (and even artistic when pictured with a macro lens as above), but there are times when I whish I could make perfectly clear ice cubes. At room temperature a certain amount of air is dissolved in water. When you cool water, the solubility of air increases (!), but only until the water starts freezing. At this point the water can no longer keep the air dissolved and a bubble is formed. Vice versa – when you boil water the solubility of air decreases and the dissolved gases escape.
When making ice cubes, the bubbles that are formed can easily escape as long as there is no ice blocking their way. This is sort of a catch 22 situation since the air expulsion is directly related to the ice formation. When making ice cubes in a normal freezer, the ice cubes are cooled from the outside, causing the air to get trapped throughout the ice cube.
Many people have thought about smart ways to achieve this (as a quick patent search shows). There are two strategies to obtain clear ice cubes. Let the gas escape while the water freezes or degas and filter the water before freezing. Icicles are a good example that when running water freezes, it normally produces very clear ice. This is utilized in commercial ice cube makers. Here a “cold finger” is exposed to water that moves. This way bubbles are carried away before they can get trapped. These ice cubes typically are ring or cup shaped. The second method is suggested many places on the net. I’ve listed them here together with some thoughts and discussion.
Degassing
Degas the water (i.e. remove the dissolved air). This is easily done by boiling the water for a couple of minutes and letting it cool again. Some webpages suggest that the process should be repeated for best results.
Slow cooling
If the water is cooled too quickly, the ice will not be able to push the impurities ahead of the freezing interface. But if an ice cube freezes from all sides it doesn’t really help as the bubbles get trapped in the middle. A drawback with slow cooling is that the solubility of gas will increase when the water is cooled and so it will allow more gas to dissolve before the water freezes. So slow cooling should probably be combined with some kind of gas tight cover.
Directional cooling
I’ve been pondering about making trays with insulated sides and cover and a metal base, thereby utilizing the fact that metals are superb heat conductors compared to plastic, wood or glass. The metal would then serve to conduct away heat from the water. Bubbles would form on the ice front, but they would probably escape, rather than become encapsuled into the ice. I’ve tried to illustrate it here:
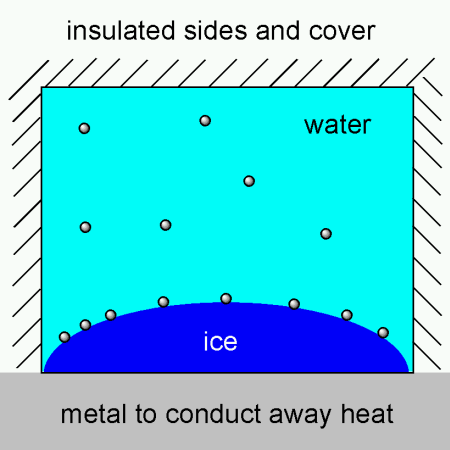
Turns out that someone has actually patented something similar where metal “fingers” are used to conduct away heat from the center, giving ring shaped ice cubes. Does anyone know if these were ever made for sale? Perhaps an ice cube tray in aluminum would work if one insulates the top so that the cubes freeze from the bottom and up, keeping the water on top free flowing so bubbles can escape.
Layer-by-layer method
There might be a simple (but time consuming) way of achieving directional cooling: By building up the ice cubes layer by layer. Once the first layer is frozen this will help freeze the next layer from the bottom up and so on. I guess layers of 1-5 mm would work, but this needs more testing. My experiments so far have not been very promising. Plenty of bubbles, even with a layer of only 2 mm.
Filtering
Particles can act as nucleation sites for air bubbles. To avoid this filter the water and make sure that all the equipment is clean. Also, don’t use a towel to try your equipment as this will probably leave small fibers behind.
Remove salts
Both tap water and bottled water contain trace amounts of salts. When water freezes these minerals are not incorporated into the ice structure. As a consequence the soluble salts will concentrate in the water that’s not yet frozen. In the end there is so little water left that the concentration of the salts becomes sufficiently high so that the freezing point of this remaining water is lower than the temperature in the freezer (meaning that this water won’t freeze). Other salts, especially calcium salts such as calcium carbonate will precipitate. And these particles can act as nucleation sites. If after boiling water there are particles present, these should be filtered away before freezing. The easiest way to get rid of salts is to use distilled water.
I’ve done a couple of experiments and it seems there is no quick fix. The water in the ice cubes pictured above was boiled for several minutes before freezing, but plenty of bubbles formed as you can see. I also tried the layer-by-layer method, but even in a thin layer of only 2-3 mm I could detect many bubbles. So clearly I need to do more experiments.
What are your experiences with making clear ice cubes?

Putting a vibrating object under the icecube tray (or other figure to be frozen) seems to work really well. Play around with different levels of vibration and such, but with very little effort you should be able to get pretty much clear ice.
I’ve always wondered about that myself… but not to the analytical extent that you have.
Years ago (when ice carvings was just a little bit cooler than it is now), we’d often purchase the huge blocks of ice necessary for carving. The blocks often came in with a ‘feather’ in the middle… or the flat straight section dead center that contained micro bubbles and impurities. If we wanted a clear sculpture, it was necessary to cut out the feather and design a sculpture around that idea.
Other companies started to offer (at a higher price) blocks without the feather. I was told that they achieved this by freezing running water… although I never took the thought process further than that.
Nice post. Very interesting.
“Turns out that someone has actually patented something similar where metal “fingers” are used to conduct away heat from the center, giving ring shaped ice cubes. Does anyone know if these were ever made for sale?”
i often see bags of ice that consists of cylinderical hollow pieces of ice they were probably made in a similar process
anon: Good suggestion. Does anyone make vibrators fit for clear ice cube production?
justin: Yes – they use “cold fingers” (cylindrical metal tips) that are cooled and immersed into water which is circulated. Some models also allow water to freeze at the end of the cold finger, giving cup shaped ice cubes.
You can always pick up an old freezer! I had an old refrigerator that used to vibrate a little and noticed all my ice cubes out of the trays were crystal clear.
Older might be better.
I’ve googled a little and found
twoseveral patents on ice cube trays coupled with vibrators!Automatic ice maker and household refrigerator equipped therewith
Icemaker
Ice maker with oscillating movement
Method for making and harvesting ice using ultrasonic vibrators
Vibrator
Seems like freezing under a partial vacuum (would take a lower temperature) would help remove dissolved gases.
how about the PH of the water, any trials with differing PH
to avoid bad smells it’s even easier to use ice cube bags like this: http://www.campingworld.com/browse/skus/index.cfm?skunum=30314 . in Europe they’re much easier to find, but at least they’re finally available in the US as well.
I am working on making very clear ice and have resorted to my Anti-Griddle. I place silicone ice trays filled with distilled water on top of the anti-griddle. The water starts to freeze from the bottom up, therefore giving the bottom a head start, and thusly pushing the air out of the top. Clear as a bell.
hi…i am doing some work on a project in which I have to establish laminar flow in a closed chamber which have 5 inlet holes and 5 outlet holes. I am pouring water into the inlets by using a water reservoir and the outlets are open to air.However there are bubbles being formed inside the chamber which are getting difficult to remove.These hinder the flow and dosen’t make the flow laminar.Could anyone suggest a way to remove these air bubbles? If there is any solvent which helps in dissolving air bubbles,it would help.
Late, but anyhow: a somewhat related problem that is rather fun is making perfect ice spheres. Reference at food for design (http://foodfordesign.blogspot.com/2008/05/perfect-ice-for-perfect-drinks.html). However, you need some new equipment that has to be stowed away in the kitchen when it’s not used (a common problem in our home). This seems to require that you have a bubble-free ice cube to begin with, though.
The final alternative is of course to obviate the ice altogether and use small rocks. Get some good looking small pebbles from the beach or a souvenir shop (commonly in science and natural historic museums), run them thoroughly in the dishwasher, and put them in the freezer. Reusable and not prone to dilute the drink. It takes all the fun out of experimenting with the ice, though 🙂
“help needed:”
Here are a few suggestions to reduce your air bubble problem. (1) make sure you are not using cold solutions. more gas can dissolve in the colder solutions (especially aqueous ones). use solutions at room temp or above. (2) what medium are your channels made in? if this medium is hydrophobic (PDMS, plastic, etc), the air bubbles will stick to the sides of the channels, because air is ‘hydrophobic.’ if you have access to a plasma oxidizer, you can use that to oxidize your channels. the bubbles will slide right through and not impede your flow. or you can adsorb a layer of protein on the channel. proteins are (generally) hydrophillic, and will accomplish the same objective as plasma oxidations.
good luck.
I played around with this (using filtered tap water) and got the best results from boiling the water twice. I have posted pictures on flickr.
I’ll probably do a bit more playing around. I’d like to try filling only every other cube (in a checkerboard pattern) to see what effect that has on the location of the cloudiness in each cube (I suspect it’ll be focused in a column or cone down the middle), but considering that the water I used came straight off the boil I don’t expect there to be any less cloudiness.
Nice pictures! And this reminds me that I have a couple of more experiments to do 🙂
I’ll bring some purified water from work to see what effect that may have.
wow! amazing!
I’m wondering how to store ice in a cooler. When ice melts in a bag or plastic container it is then surrounded by water – is it better to drain the water and leave the ice “dry” in the cooler or will the water help keep it frozen longer?
My guess is that it’s best to drain the water – otherwise it will act as an efficient transporter of heat from the sides of the container to ice cubes. Air on the other hand is a pretty good insulator.
You say that particles act as nucleation sites. But don’t you actually want the air to nucleate as soon as possible before it freezes to get it out?