Here’s some pictures and a video of my first experiments with sodium alginate and spherification. I used sodium alginate from the Texturas series and calcium chloride from a drug store. Needless to say, I’m very fascinated by the texture and the whole process. I have blogged about the chemistry behind previously.
Materials used:
2.0 g sodium alginate
200 g water (with low calcium content!)
50 g blueberry syrup
2.5 g calcium chloride
500 g water
Procedure:
2 g sodium alginate and 200 g water were mixed vigourously in blender. The mixture was then left to stand for some hours to get rid of the air bubbles. 50 g blueberry syrup was then added to the sodium alginate solution. A calcium chloride bath was prepared by dissolving 2.5 g calcium chloride in 500 g water. The sodium alginate/blueberry mixture was dripped into the calcium chloride bath using a plastic syringe with a steel cannula. After 1-3 min the pearls were removed and rinsed with water.
More detailed procedure with pictures and video:
I had to obtain a scale with a 0.1 g accuracy to weigh out 2.0 g of sodium alginate (my first experiments using a normal kitchen scale failed). The model I got cost about $100 and is inteded for school laboratories.

I used a blender to dissolve sodium alginate in water. This incorporates a lot of air in the mixture which we don’t want. It could possibly be avoided by using an immersion blender/mixer. However, I just left the alginate solution on the bench and after 3-4 hours the air bubbles had all escaped from the solution.

Plastic syringes and cannulas can be obtained from your local drug store or pharmacist. I found it was easier to produce evenly sized drops with a sharp cannula (CAREFULL!) than with just the plastic tip of the syringe. This of course depends on the viscosity of the solution. By thickening (with xanthan for instance) you can produce larger drops.
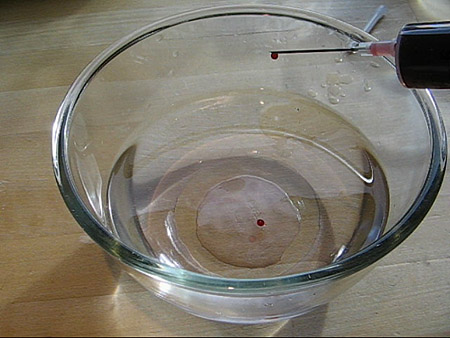
After 1-3 min the spheres were removed from the calcium chloride solution and rinsed with clean water. I dried the spheres carefully using a kitchen towel or paper.

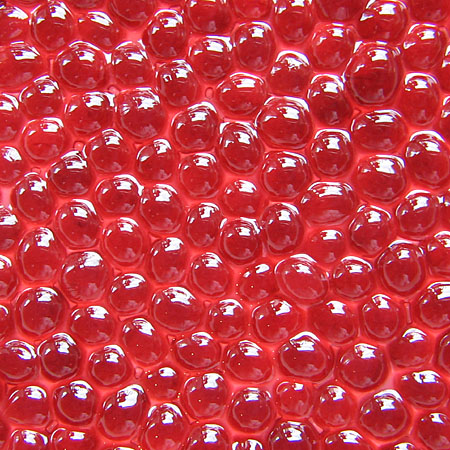
Definitely looks like caviar when presented on a spoon like this!

Larger spheres were made by filling a small measuring spoon with the alginate mixture (I used a syringe for this so the outsides of the spoon would not be covered with alginate solution) and carefully emptied it into the calcium chloride bath. It takes some trial and error to achieve good results.

The spheres are suprisingly robust and can be handled without rupturing.

If cut with a knife, the spheres rupture and the liquid contents flows out.

The small spheres didn’t taste much, so I could have added more blueberry syrup. The large spheres however had a nice taste. The surprise element when they rupture in your mouth is very nice!

You might be able to shorten the time needed to get rid of air bubbles by bubbling helium through the blended alginate solution? (It works reasonably well to degas organic and aqueous solutions for HPLC and other chromatography methods) A narrow pore filter should work too, but it might be easier to get a small tank of helium?
“[…] I could have added more blueberry syrup.”
Does that mean just more blueberry syrup, or would one have to recalculate all the ingredients?
I am curious about a few things:
1. If left out, what happens to the spheres? Does the outside dry out and harden? Does the inside eventually evaporate through the “crust?” How long will they hold their shapes?
2. If heat or cold were applied, what would happen to the spheres? For instance, freezing them, or using a kitchen torch on them? At what temperature would they lose their shape? What if the spheres were dropped into boiling water?
hi. i’m writing an article for a newspaper about molecular gastronomy in the home. anybody out there who’ve tried it (notably khymos in this blog) whom i could call to ask a few questions? thanks a million. beppi
Barney:
I’m afraid bubling some other gas thorough wouldn’t really help. The problem is not dissolved gas, but gas bubbles trapped in a viscous liquid. However, a couple of freeze-pump-thaw cycles would do the job 🙂
Nils:
I would have substituted some of the water, keeping the total amount of liquid the same.
Barzelay:
The liquid interior will slowly dissipate through the soft alginate skin. I’m not quite sure how the alginate spheres would react to cooling/heating.
Beppi:
Feel free to contact me on webmaster (at) khymos (dot) com.
[…] Playing with your food: Sodium alginate pearls […]
fast way to get rid of foam is to slowly pour the foamy mix through a fine-meshed strainer
misuse of alginates can be atrocious, we used to do some moonlighting in a bakery plant in highschool, and the alginate job (making bright-colored sticky blobs of artifically-flavored candied-fruit substitute) was the most despised asignment (disgusting smell, floor and walls and all other surface totally sticky) – whereas baking pastries or soaking raisins in rum was the favorite job
I’m not sure how much I believe the explanation, but people seemed to think that enough helium would displace any entrained/dissolved air and then the helium would outgas on its own (?).
A kitchen set up with a manifold for freeze-pump-thaw degassing would be a lot of fun (though odds of someone sooner or later condensing some liquid oxygen seem pretty good).
Maybe a sonicator (for jewelry cleaning, etc.) would be another easily available device that would aid in degassing?
Do you think you could make worm/noodle-like capsules by slowly extruding the alginate solution from the cannula into the CaCl2 solution?
Pouring the mixture through a fine-meshed strainer sounds like a good idea which I will try.
Yes – if you lower the syringe into the CaCl2 bath and apply a constant pressure you get a noodle like structure.
I’m trying this for the first time at home, but eventually for restaurant use. Any idea as to how long they stay stable under refrigeration? Secondly, do you know how alcohol might effect the physical properties of the spheres?
They’re stable for hours, but not for days – the liquid content will slowly leak out. Alcohol works fine, but dissolve alginate in pure water first. Aim at the same total concentration of alginate.
[…] up: Ales’s Glorious Stinking Anchovies & Martin Lershch’s Blueberry Pearls […]
I’m from New Zealand and have been having a hard time trying to find calcium chloride!!!. There is one place that can get it in for me but its going to cost $100NZ for 10ml. Is this what you are paying for your calcium chloride and do you know of a place that I could get it?
I would visit a pharmacy or a drug store and see what they have. You would want CaCl2 as a solid, not as a solution (the price you quote seems to be for a solution). For example, my Fluka catalog lists 1 kg calcium chloride dihydrate for €22.40 (with a purity conforming to the European Pharmacopeia requirements).
Just for interest, did you know that this technology was used for commercial production of pie fillings in the 70’s and 80’s. Batchelors Foods in the UK had a range of pie fillings under the ‘Pack A Pie’ brand, and the spherification technique described above was used for blackberry and cherry versions. Other varients included apple (mixing fruit puree, alginate and calcium chloride, and sheeting out), and Apricot (similar to apple, but applying into a mould). The products were good, but never replaced real fruit!
Yes – and AFAIK McDonald’s still uses alginate for their apple pie filling (correct me if I’m wrong – I haven’t checked this up). Also, the little piece of “red pepper” in olives is often not what it looks like but a rather a red pepper purée that’s been gelled with alginate and caclsium ions.
Very interesting! Thank you for sharing, and definitely find it helpful.
However, I am in Kuala Lumpur and find it very difficult to obtain these chemicals.
i have try several times the recipes fro the texturas site but nothing realy happens except a masse from the alginate solution…….. why? the alginate and the sodium are not from texturas but from a drug store… can be just old???…..
thanks for your blog khymos or Χυμός
Has anyone ever tried to do this with something other than fruit..such as chocolate? in that case, would you recommend something other than CaCl2, maybe gluconolactate? i’m not sure what the free calcium ions would do to the concentrated chocolate.
thanks
Hi
I’m knew to this & tried to make champagne caviar by replacing the water & blueberry syrup with champagne. I boiled off the alcohol in the champagne & followed the recipe as per the blueberry ones but they didn’t quite set properly. Any suggestions………….????
Hi to Femmie this info may be a bit late but I have just ordered a kg of calcium chloride for $45 from http://www.totallab.co.nz/
CaCl2 can be purchased quite cheaply in 3 kg pails from pool supply shops or other retailers that sell swimming pool chemicals. Usually referred to as a water hardner.
This CaCl2 is obviuously of a technical quality, not intended for consumption. They price suggests that it’s not very pure. In the experiment above I only used 2.5 g. My suggestion would be to obtain a smaller quantity from a drug store or a pharmacy (or one of the MG suppliers).
Since you, family, friends, customers, will consume foods created with various chemicals it is absolutely necessary that these chemical ingredients be of food grade. It would be extremely unwise to purchase chemicals suitable for use in swimming pools or high school chemistry experiments and use them in the preparation of food.
There are many places on line to purchase food grade components. In most cases only very small quantities of these components are used so they will last for many “experiments” at home.
I found http://www.lepicerie.com/ to be a great resource for the novice molecular gastronomist.
Are you sure these chemicals are safe to eat?
The chemicals are definitely safe to eat. Calcium Chloride or Calcium Lactate are both sold as a calcium supplement. You need the calcium, chloride doesn’t harm you, as it is in sodium chloride (salt). Lactate is simply lactic acid, which your body produces naturally. Sodium Alginate. With sodium, once again, you can have that as it is in table salt. Alginate is from brown algae and also safe.
I am doing a high school science project. This experiment is 100% the one I would like to do. But I am confused how it works. I understand the process to create the spheres, but how does it happen?! I need to figure this information out to explain in my project not just the how but the why. I am very excited to try this because I will be the one bringing something edible, not a silly volcano. As I aspire to become a chef myself and possibly dive off the boat into the unknown field of Molecular Gastronomy any information and explainations would be happily recieved! Email if possible: Neptunes_warrior2003@yahoo.com
I’ve written a little about the chemistry here:
http://blog.khymos.org/2006/09/17/video-on-alginates/
[…] website can explain it better than I can: blog.khymos.org » Blog Archive » First experiments with sodium alginate __________________ "Boldness has genius, power, and magic in it." Johann Wolfgang Von […]
[…] Let me know how it turns out. I was going to try something similar with blueberry syrup, the recipe is here: blog.khymos.org » Blog Archive » First experiments with sodium alginate […]
How much is a gram in teaspoons? I thought I measured it out right but it did not come out. Does the size of the dropper matter? I what to use this for Bible School in June and am starting now to make sure it works.
1 teaspoon = 5 mL = 3.5 g sodium alginate (measured for Algin from the Texturas series)
Note that most normal teaspoons hold smaller volumes than a “measuring teaspoon”.
An other way to degas is to apply a lower pressure to the mixture e.g. by putting it in a bottle and then sucking on it with a vacuum cleaner… 🙂
Is this safe to serve to people?
Yes 🙂
I use this method in research to encapsulate pieces of plants to make “synthetic seeds”. I believe that I can answer some of the persisting questions:
On removing trapped air bubbles:
Don’t use a blender. The alginate can be melted in the water just like gelatin can. We add ours to the water in a pyrex jar, then put it in the autoclave (ie – pressure canner). We cook we cook ours at 250F for about 14 min. It can be stirred afterwards. If you break up remaining clumps while it is still hot, they should melt. You can also strain it.
On storage:
Alginate beads can be stored in refrigeration without ill effects. I would recommend a storage period appropriate for the contents. Also, drying out will occur unless the beads are stored in a sealed container. You can also splash on a little water just to make sure.
I might just have to try some culinary applications myself.
This was an very inspiring article; I’m eager to begin working with these spheres of blueberry – but where do I find sodium alginate?
Preferably in a country that is member of the European Union, but it would be just as nice to know where you, Martin, get your chemicals from.
I have been making caviar for a while. i’ve used coca cola, strawberry, orange, vinegar, and peas. I was experimenting with a recipe for cubes, and it didn’t work. I used:
20g sugar
10g dextrin
170g orange juice
3g sodium alginate
1tsp lemon juice
2g calcium lactate.
I blended the dextrin, lemon juice, calcium lactate, and half of the juice. then i blended the sugar, alignate, and the other half of the fruit juice. then i blended it all together and let it sit for half an hour, just like the recipe said. after about 40 minutes the mixture didn’t solidify enough for me to cut it into cubes. I was wonder if you could please tell me what i did wrong here, or another recipe that worked for you. this will be greatly appreciated!
-Mark
If you want to make cubes, surely you need to think about making a gel?? Agar would be perfect for this. The alginate works because it forms a membrane around the liquid and probably increasing the surface tension of the liquid inside.
Thank you, Chris. I’ll buy some agar and try it and i’ll post the results.
Mark – perhaps you should adjust your pH? Orange juice can be quite acidic. Read more about this on page 45 in “Texture – A hydrocolloid recipe collection”. I assume that the alginate is properly dispersed (no lumps) and hydrated (left over night in fridge).
The alginate was dispersed and i didn’t hydrate but now I will. And I’ll try some other beverage. Thank you so much! I’ll let you know how it goes!
I’d love for someone to explain to me how to do adjust this recipe with chocolate. I know it can be done b/c I’ve seen it, but I just don’t know the ingredients to use and quantities to make it happen.
Thanks!
Anybody made agar agar noodles, ratios, ph concerns in a pvc tube or out? If in the tube what is the easiest way to get the noodle out, syringe or by other means?
Any help with this would be rad.
Thankyou
Check out “Texture – A hydrocolloid recipe collection” at https://khymos.org/recipe-collection.php. I used agar noodles for the front picture and you can find several recipes in the collection.
I’m trying this for the first time at home, but eventually for restaurant use. Any idea as to how long they stay stable under refrigeration? Secondly, do you know how alcohol might effect the physical properties of the spheres?
I surprised no one mentioned it but a quick way to remove bubble from the solution is place it under a vacuum. We common do that in the lab for degas solutions for either chromatography applications or preparing electrophoresis gels. In the lab, we use nothing more than a rubber stopper with a hole in it to which we attach a tubing that leads to a vacuum source. At home you can use any vacuum sealer that has an attachment to seal jars.
Three things to note. First don’t use any jars that have deep scratches, this greatly lowers the structural strength and you risk implosion of the bottle. second, since it is a viscous liquid, it may foam up a bit when under the vacuum as gas escapes, so fill your container more than 2/3 full to avoid a potential mess. Third, always gentle release the vacuum to avoid introducing new bubbles.
what kind of failed did you have when you did use kitchen scale? Because mine doesnt make a balls thing when it drop into calcium bath… ?? it just spread out and dissolve with it…
cheers
thx
[…] step was to find out what to do, I found a Blog (here) which had a good first look at how to use these two chemicals, I modified it slightly to suit my […]
Here’s a quick comment to “Jo” about using chocolate. In the first post you were worried about using the calcium solution with chocolate… to do this experiment/reaction, you must have the calcium present in order to exchange ions and create the “cross-linking” which in effect is the gel itself. It would be important that the solution you drop it in would be the one to have calcium (try milk or cream).
One minor set-back about using chocolate; chocolate and water are not usually the best of friends.
For those of you out there who work with chocolate in pastry kitchens, you all know this very well:
small amount of water + melted chocolate = big mess of thick solid icky (aka seizing up of the chocolate)
However… if you use a larger quantity of water (with algenate already dissoved in it) and add the melted chocolate, mix to encorporate, then drop it into milk… it might just work. The reason the larger amount of water works is because it will dissolve all of the sugars and create a liquid again (example: Genache, cream + melted chocolate).
Good luck!!!