Here’s some pictures and a video of my first experiments with sodium alginate and spherification. I used sodium alginate from the Texturas series and calcium chloride from a drug store. Needless to say, I’m very fascinated by the texture and the whole process. I have blogged about the chemistry behind previously.
Materials used:
2.0 g sodium alginate
200 g water (with low calcium content!)
50 g blueberry syrup
2.5 g calcium chloride
500 g water
Procedure:
2 g sodium alginate and 200 g water were mixed vigourously in blender. The mixture was then left to stand for some hours to get rid of the air bubbles. 50 g blueberry syrup was then added to the sodium alginate solution. A calcium chloride bath was prepared by dissolving 2.5 g calcium chloride in 500 g water. The sodium alginate/blueberry mixture was dripped into the calcium chloride bath using a plastic syringe with a steel cannula. After 1-3 min the pearls were removed and rinsed with water.
More detailed procedure with pictures and video:
I had to obtain a scale with a 0.1 g accuracy to weigh out 2.0 g of sodium alginate (my first experiments using a normal kitchen scale failed). The model I got cost about $100 and is inteded for school laboratories.

I used a blender to dissolve sodium alginate in water. This incorporates a lot of air in the mixture which we don’t want. It could possibly be avoided by using an immersion blender/mixer. However, I just left the alginate solution on the bench and after 3-4 hours the air bubbles had all escaped from the solution.

Plastic syringes and cannulas can be obtained from your local drug store or pharmacist. I found it was easier to produce evenly sized drops with a sharp cannula (CAREFULL!) than with just the plastic tip of the syringe. This of course depends on the viscosity of the solution. By thickening (with xanthan for instance) you can produce larger drops.
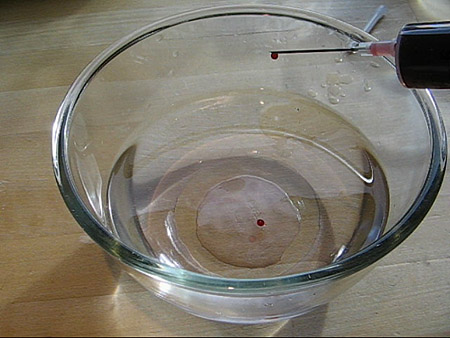
After 1-3 min the spheres were removed from the calcium chloride solution and rinsed with clean water. I dried the spheres carefully using a kitchen towel or paper.

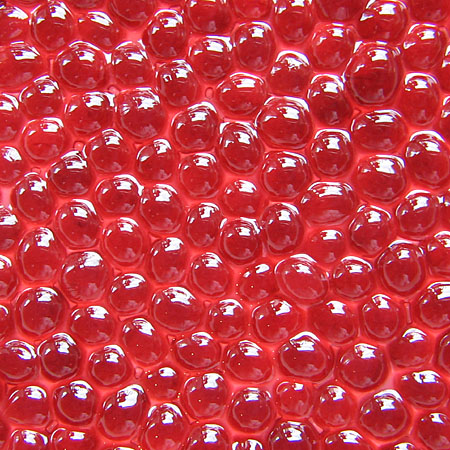
Definitely looks like caviar when presented on a spoon like this!

Larger spheres were made by filling a small measuring spoon with the alginate mixture (I used a syringe for this so the outsides of the spoon would not be covered with alginate solution) and carefully emptied it into the calcium chloride bath. It takes some trial and error to achieve good results.

The spheres are suprisingly robust and can be handled without rupturing.

If cut with a knife, the spheres rupture and the liquid contents flows out.

The small spheres didn’t taste much, so I could have added more blueberry syrup. The large spheres however had a nice taste. The surprise element when they rupture in your mouth is very nice!

Martin, apologies for the “necromancy” of an old thread.
You advise to use trisodium citrate to adjust pH in acidic juices (btw, its page 52 in your pamphlet, not 45 😉 ). Are there easy substitutes? I have no idea where to find trisodium citrate here in the UK.
Thanks
Robert
So so cool. Thanks for the recipes, ideas, and science. Was watching an old “Iron Chef” with Chef Cantu from Moto…..challange beets….it was the Iron Chef that used this tech however to make faux salmon roe.
Just stepping into the molecular ring and this sounds like it is going to be fun.
I am new to all this science stuff so please bear with me…
I am interested in using spherification in order to create a starter and wondered…. once made are you able to gently heat an ‘orb’ of liquid?? Maybe in a low temp water bath??
Maybe I am one the wrong path and need to use another method to achieve the same goal??
Matt: Yes – you should be able to heat it carefully. The calcium-alginate gel is heat stable.
Hi there folks!!
Is there a commercial name for alginate, or do I just walk into the pharmacy and ask for alginate?
Thanks!
Philip
You could try. If it doesn’t ring any bells you may also ask for “sodium alginate” which is more correct. There are branded names as well: Algin (from Texturas), Algizoon (from texturePro), Gelesfera A (from Sosa) – just to mention a few.
Thanks Martin… wish you could have seen the face of the assistant at the chemists here… I am sure they did not know whether I wanted to use it or sniff it!
I found guar gum… can I replace that for the alginate?
In the meanwhile I have discovered a distributor for Texturas in South Africa, but they charge a king’s randsom for the product…
If the chemists have some biochemical training they might actually have heard about alginate beads gelled with calcium. Amongs many things such beads are used to immobilize microorganisms such as yeast.
Anyhow – if your local supplier is too expensive, why not order from someone else on this list: http://blog.khymos.org/links/suppliers/
I tried to make spheres from soy sauce using the following formula:
1 g sodium alginate
100 g water
25 g soy sauce
1.25 g calcium chloride
250 g water
I combined the alginate and eater, mixed well with an immersion blennder, waited several hours, added the soy sauce to the solution. then prepared the calcium chloride bath. When I injected the alginate solution into the bath nothing happened. This was my first attempt at this and I don’t know if my formula is bad, is soy is not a good substance for this process or if my scale was inaccurate.
Can someone review the above and give me dome idea as to where I went wrong.
Joe,
the alginate blend should be at least 2% weight by volume. So use 2 grams of Na-Alginate in 100g water. You dont even need to use a blender to dissolve it. At about 40 degC and continuous stirring, alginate will dissolve in about 20-30 minutes. There will be less bubbles hence, less waiting time.
Also the calcium chloride drop should also be at least 2% (w/v).
Hope this helps
I followed a cola recipe in Martin’s free download hydrocolloid recipe book and everything worked perfectly. I substituted strawberry soda for the cola and ended with something that resembled Flying Fish roe. I had one down side and that was due to the calcium chloride bath. The spheres had a salty taste on the outside when you first put them in your mouth. I was using food grade calcium chloride and I even rinsed off the beads and let them soak in a larger bowl of water and they still had that salty taste on the outside.
Any advice?
Hi! Just stumbled upon your blog randomly and I would like to say awesome post and video. Looks dead easy; now I need to get my hands on some sodium alginate and calcium chloride 🙂
Thanks for sharing
Hi,
I am looking to purchase the sodium alginate for a non-edible science experiment. Non-edible
sodium alginate is way cheaper. Does anyone know if it will still work in an experiment? I’m making Instant Worms for second graders! Please help!!!
i have a feeling this has been asked but
where do you buy food grade sodium alginate?
i not only want to use it for food but also for textile use.
can you get it at a grocery store, if so what section?
and what is the average cost for a decent amount.
thx,
E Deng
PS i want to avoid buying off the internet
There is a list of (internet) suppliers available here: http://blog.khymos.org/links/suppliers/
Apart from these I’d suggest you check out local gourmet shops where you live. In Europe some gourmet/specialty food shops do stock the Texturas series or similar products from texturePro, Kalys or other vendors.
Regarding prices, my best advice is to check what the internet suppliers charge. This should give you a good idea of what the selling price is.
I have a local supplier selling Sodium Alginate, food grade. But when I tried to order, he started asking about mesh and stuff. Any idea what is that?
When mixing the sodium alginate into my desired puree, (Watermelon/Ginger, Lychee/Elderflower.) the alginate isnt entirely resolving, and when I tried it in my vita-mix on the lowest speed, it thickened quickly. Advice? The ratio’s were 2g Sodium alginate to 200 g liquid.
When mixing the sodium alginate into my desired puree, (Watermelon/Ginger, Lychee/Elderflower.) the alginate isnt entirely dissolving, and when I tried it in my vita-mix on the lowest speed, it thickened quickly. Advice? The ratio’s were 2g Sodium alginate to 200 g liquid.