Cookware made from cast iron has a reputation for keeping food warm for a long time. Is that really true? Best way to find out is by an experiment. I decided to compare a cast iron pot with one of stainless steel. These are the pots I used:

For the first experiment I filled them each with 2,5 L of water, put the lids on and brought both to the boil and let them boil for a minute so the pot itself would be warm throughout. Then both were placed on cork plates and left to cool. The temperature probe was carefully inserted under the lid in order to reduce the heat loss, and removed once the temperature had stabilized. For the second experiment 5 L of water were used. The measured temperatures are shown in the graph.
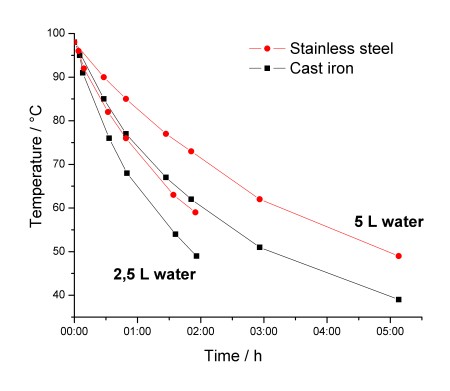
Contrary to what I had expected, the stainless steel pot keeps water warmer! After approximately 1,5 hours there is a 10 °C difference between the two. As expected, when using 5 L of water, it stays warm longer. Physical data for the two pots are given in the following table:
| Cast iron | Stainless steel | |
|---|---|---|
| Volume | 6 L | 6 L |
| Diameter | 27,9 cm | 25,0 cm |
| Height | 11,5 cm | 14,5 cm |
| Surface area (top+sides) |
1619 cm2 | 1629 cm2 |
| Surface area in contact with 5 L water |
1301 cm2 | 1286 cm2 |
| Weight | 6,1 kg | 2,3 kg |
| Wall thickness | ~4 mm | <1 mm |
| Heat capacity of pan | 2,8 kJ/K | 1,2 kJ/K |
| Thermal conductivity | 80 Wm-1K-1 | 16 Wm-1K-1 |
| Thermal diffusivity | 22 x 10-6 m2/s | 4.3 x 10-6 m2/s |
| Emissivity | 0.95 | 0.07 |
The heat capacity of the cast iron pot is more than double that of the stainless steel pot. But this is negligible compared to the heat capacity of water: 10.5 kJ/K (2,5 L) and 20,9 kJ/K (5,0 L). Also, there is only a small difference in their surface area which cannot explain the large difference in temperature loss observed.
This leaves me with two eplanations:
My guess is that the difference in emissivity is more important (but please correct me if I’m wrong). With an infrared thermometer, one should therefore be able to measure a difference between pots of cast iron and polished stainless steel (even though they’re at the same temperature!) due to the difference in emissivity. Any one who can do the experiment and report back?
Conclusion: There are many good reasons to use cast iron, but keeping food warm is not one of them!

Interesting. My understanding is that cast iron’s biggest attraction is its higher specific heat capacity, which keeps it from dropping in temp. as cold food is added to it. Not that it keeps food warmer after removed from the heat.
It might be worth while to try this out with an enameled cast iron pot too.
If the reason is emittivity then covering the pot in aluminum foil might make it stay hot longer because the radiation would be reflected back to the pot
Your cast-iron pot is most likely made from cast-iron, but you stainless steel is not: the pot will have an aluminum or copper core in the base plate. When I switched from low cost stainless steel pots to “˜commercial grade’ ones I noticed a much higher heat capacity. Oil will continue to boil even when taken from the burner. The stainless steel pots made by the Finish company OPA (http://www.opa.fi/english/products/pro.htm) have an aluminum “˜core’ in form of a disk placed between two sheets of stainless steel which are welded at the top(!) of the pot, leaving a sealed space of “˜air’ in-between the walls of the pot. I’ve seen a cut-open model of this construction while visiting their company store last year. Before the redesign of their web site, that model was even shown there. All I want to note is that you have to be really careful when doing measurements with “˜normal’ stainless steel pots.
DrObviousSo:
There is a very small difference in specific heat capacity between cast iron and stainless steel (0,46 J/g*K vs. 0,50 J/g*K). In the table I calculated the heat capacity for the two pots (based on their masses). The cast iron pot has a heat capacity more than double that of the stainless steel pot. But if your pot is filled with water, the heat capacity of the pot itself is negligible.
Felix:
Yes – I would also expect that covering cast iron with aluminum foil should reduce the heat radiation from the pot. Should the shiny side of the aluminum foil face inwards or outwards?
Mirko Junge:
You’re right that the base of my stainless steel pan is a compound base. In fact it has a compound base with 5 layers. The three middle layers would be aluminum for heat distribution and some ferrous material to allow use on induction tops (unfortunately I can’t find the excat information on the net now). However, since the the pots were placed on cork plates, the properties of the base shouldn’t influence the results significantly. I’m quite sure that the sides and the lid are made of solid stainless steel. The pot is manufactured by the Iittala/Hackman/Høyang-Polaris consortium and is part of their “All steel” series.
I guess the shiny side should point inwards – the shinier the better it reflects (if we expect that the foil has the same behaviour for infrared radiation that it has for visible radiation)
OK -I could see stainless composite keeping heat longer- however us Cast Iron fanatics don’t look for that property so much, if at all. We like the even heat distribution, the lack of a possible carcinogen ( teflon ) – and the no stick surface of a good seasoned pan or pot.
We ditched all our other pots and pans for good old cast iron – except for our pyrex glassware. Thats about as non reactive as you can get.
Rob
Cast iron has better thermal conductivity than stainless steel. The surrounding air is cooler than the water so it begins to cool with the passage of time. Since cast iron is a better conductor of heat, it transfers the heat of the water to the surroundings better than stainless steel. If you had put the water into a large styrofoam container it would have stayed warm even longer! Yet if you cook in styrofoam it will disintigrate where it comes into contact with the burner. But the foam will be fine elsewhere. Why? Because styrofoam has poor thermal conductivity. Likewise cooking in a stainless steel pot or pan may scorch or burn the contents in the vicinity of contact with the burner because it too has poor thermal conductivity. That is why an aluminum or copper base is added to a stainless steel pot/pan. Cast iron does have higher heat capacity than stainless steel. Also it is denser increasing this effect. Also it is thicker (more volume, the pot that is) yet again increasing this effect. This extra heat stored in the pot/pan in conjunction with the higher thermal conductivity acts as a buffer to protect against hot spots burning or scorching food in the pan. You could aim a propane torch at the bottom of a cast iron skillet and cook just fine. With a stainless steel pan you’d likely punch a hole through the pan.
the pans are not the same shape. This is the most likely cause of variance in heat transfer times. The wider pan will expose more surface area than the narrow pan, allowing greater heat transfer. The ideal shape to minimize heat loss is a sphere. (least surface area per volume). Also, a large heavy pan acts as a heat-sink, pulling heat out of the water and transfering it to the surrounding air.
The surface areas are in fact almost the same. From the table: Surface area (top+sides) 1619 cm2 vs. 1629 cm2.
It’s true that the pans have different mass and different heat capacities, but this is negligible compared to the heat capacity of water.
In conclusion I still think that it’s the difference in emissivity which is the main reason for why the stainless steel pan keeps water warmer.
(I realize now that there is one parameter which I forgot to include in the table – the thickness of the walls/lid – the cast iron pan for sure is much thicker, but I’ll measure them both and report back)
you need to calculate the surface area of the actual cyllinder of water within the pan,(the area of the water that actually touches the pan) not the area of the pan itself. Iron, having greater thermal capacity, as well as conductivity, will draw more heat up out of the water into the top portion of the pot, increasing the effective surface area for heat radiation. To remove these variables, it would be necessairy to have two pots of identical shape and volume, and to fill them both to capacity.
chris: I’ve updated the table now with wall thickness and the surface area of the water that is in contact with the pan. I can’t see how this should explain the large difference observed for keeping water warm.
I have heard that the quality of cast iron pots n pans these days is not the same quality it has been renowned for in the past, how best would one determine/know they are buying high/good quality iron?
the cast iron post is a more efficient condutor of heat between the air and the pot itself (combination of conduction and convection). This is due to the fact the cast iron surface is rougher than a polished stainless surface, so if you look on a micro level there is a much greater surface area for the air to come in conctact with.
I doubt radiation would be a factor. radiation only comes in to play when the difference in surface temperatures gets closer to 1000K not 100. You could test this by quickly placing a sheet of glass next to the pot and see if you can feel any warmth thru the glass. This will seperate the conduction/convection with the radiation.
Awesome experiment!
I would try the experiment again this way: preheat both pots for say 5 minutes. Then add already boiling water into the pots.
I am thinking that the cast iron will get hotter and stay hotter longer. No science, just guessing.
For a computation of air convection losses on the pot look at the online book “Renewable energy resources” by Twidell & Weir, Example 3.3
http://books.google.com/books?id=CipkYGCrecEC&pg=PA58
Rescaling to the pots of Martin’s experiment it gives 83.5W and 81.5W for cast iron and SS at 100°C. These values should be taken as indicative (say with 5% uncertainty).
The blackbody radiation terms, according to the reported emissivities, are 135W and 10W.
Summing together convection+radiaton, we have total power losses of 218.5W for cast iron and 91.5W for SS.
The thermal capacities added by the pot materials are instead negligible (+13% and +5.5%) compared to the 5 L of water.
The calculated cool-down time constants are 2.4 hours (2h25min) for iron and 5.37 hours (5h22min) for SS, both filled with 5 L of water. The meaning of the time constant is the following: on Martin’s graph draw a line tangent to the curve at time=0 and find the the time this line takes to reach ambient (20°C) temperature.
Hoping to have clarified a bit the matter