
Some years ago, a group of researches studied the formation of lightstruck flavor in beer (Chem. Eur. J. 2001, 4554). They found that isohumulones, compounds contributing to the bitter taste of beer, decomposed when exposed to ultraviolet light. In a recent blogpost, Harold McGee elaborates on this and it turns out that the way this happens is even more complex than first anticipated. The researchers (J. Agric. Food Chem, 2006, 6123) found that riboflavin (vitamin B2) acts as a photosensitizer in beer (and in olive oil, milk and butter) which catalyzes the conversion of oxgyen to a more reactive type of oxygen (singlet oxygen). This oxygen then “destroys” isohumulone and in the process radicals are formed.
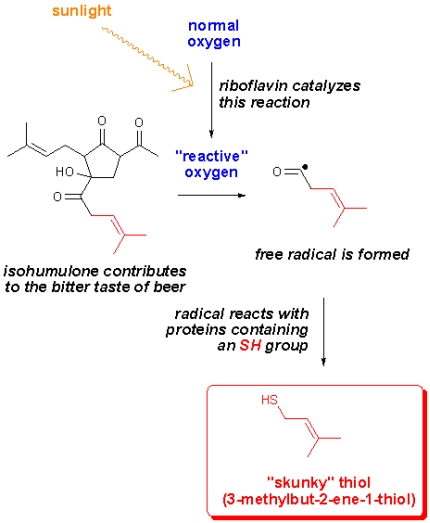
As shown in the figure, the radical reacts with sulfur containing proteins, thereby forming a thiol called 3-methylbut-2-ene-1-thiol or just MBT for short. The amazing thing about this compound is that we can smell it at concentrations as low as a few parts per billion (ppb). The perhaps not-so-amazing thing is that this compound gives beer a “skunky” aroma. Obviously one would want to avoid this, and that’s why beer is sold in dark brown glass bottles that act as the beer’s own sunglasses. Canned beer of course will not go skunky (well not until it’s poured into a glass and served outside in bright sunlight – that will turn any beer skunky within minutes).
Unfortunately however, not all beer is sold in dark bottles! One well known brand is shown in the picture below…

And yes – as you might have figured out, 3-methylbut-2-ene-1-thiol is present in Corona beer (and other brands sold in clear bottles, to a lesser extent MBT is also found in green bottled beer). For some references to “skunky” off flavours in beer check out these links: here, here and here. The ubiquitious slice of lime served with Corona beer is nothing but clever marketing since it helps camouflage the smelly thiol formed! (but how well does lime actually camouflage the thiol aroma?)
The take home message is: keep your olive oil, milk, butter and beer away from sunlight!

[…] Over at khymos.org, a site dedicated to “molecular gastronomy” and the science of cooking, has put together a very nice explanation of the process, including diagrams for those of us who have already been drin…I mean, those of us without a chemistry background. […]
I heard that the glass of clear beer bottles contains a high amount of metal oxides (vanadiumoxide?) which act as UV filter. Its the same effect as with the brown and green glas (ironoxides?).
But after many experiments I found a nearly perfect place for storing beer: the fridge…
[…] Lightstruck flavor in beer GREAT summation of the chemical reactions behind skunkiness. (tags: alcohol beer food brewing chemistry) […]
[…] to brown glass for its bottles; I hadn’t bought a Sam’s for years for this reason. Clear glass is bad for beer. But you already knew […]
Gawd, when will people learn about the atrocity of green glass? I do not even give beers in green glass a chance anymore. Usually these beers are a bit too strong for my palette anyway. I prefer smooth, crisp, micro-brewed ales in brown bottles. Gawd!